LNP delivers mRNA to lower cholesterol
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
LNP delivers mRNA to lower cholesterol
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
LNP delivers mRNA to lower cholesterol. Lipid nanoparticles deliver single-base editing system mRNA to reduce cholesterol once and for all.
On May 7, 2019, Verve Therapeutics received US$58.5 million in Series A financing from companies such as Google.
It plans to use the funds to develop a once-and-for-all gene editing therapy for heart disease.
Through lipid nanoparticles (LNP), CRISPR The single-base editing system is delivered to the cell to realize the prevention and treatment of heart disease.
On May 7, 2019, Verve Therapeutics received US$58.5 million in Series A financing from companies such as Google. It plans to use the funds to develop a once-and-for-all gene editing therapy for heart disease.
Through lipid nanoparticles (LNP), CRISPR The single-base editing system is delivered to the cell to realize the prevention and treatment of heart disease.
After the news was announced, it attracted widespread attention and reports.
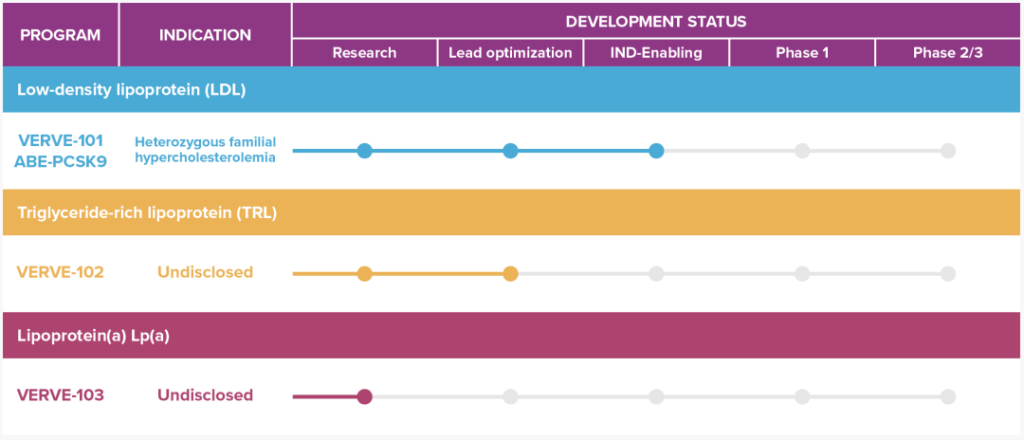
At the JPMorgan Chase Healthcare Conference that is currently being held, Professor Sekar Kathiresan, the founder of Verve Therapeutics, announced that he will launch a clinical trial of gene therapy for heterozygous familial hypercholesterolemia (HeFH) based on single-base editing technology.
 Prof. Sekar Kathiresan
Prof. Sekar Kathiresan
The therapy, called VERVE-101, uses lipid nanoparticles (LNP) to deliver a single-base editor to single-base editing the PCSK9 gene to inactivate it, thereby reducing cholesterol levels and treating heterozygous familial sex.
Hypercholesterolemia (HeFH). There are genetic mutations in the liver of people with HeFH, which can lead to high cholesterol levels, and lead to heart attacks or strokes early in life, which seriously threaten life and health.
The disease is a relatively common genetic disease, affecting one in every 200-500 people worldwide.
At present, Praluent produced by Biopharma and Repatha produced by Amgen both target PCSK9 to reduce cholesterol and can treat familial hypercholesterolemia, but these two drugs need to be injected every two to four weeks. , The annual cost is more than 14,000 US dollars, it is difficult to be widely used.
But if drugs based on CRISPR gene therapy can be effectively prevented and treated for life with a one-time injection, it will obviously save a lot of money, and this product that brings real benefits to patients will also be fully reimbursed.
Verve Therapeutics reported experimental data on monkeys in June last year.
Two weeks after the injection, the PCSK9 gene expression in the liver cells was reduced by 67%, the PCSK9 protein in the blood was reduced by 89%, and the “bad cholesterol” level was reduced by 61%.
Now the experiment has been over for more than 6 months, and the treatment level is still very good.
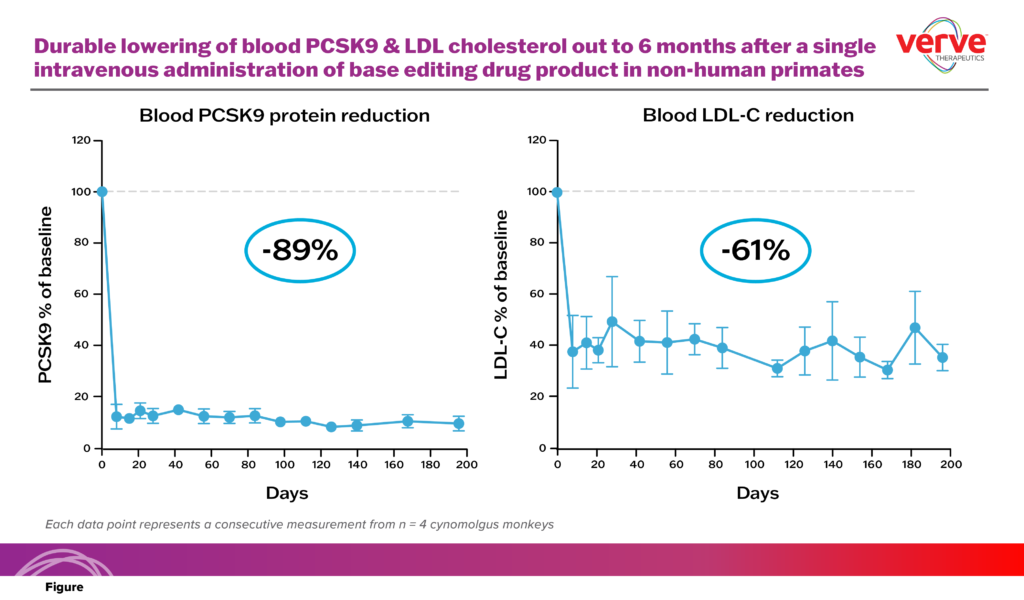
At this year’s JP Morgan Medical Conference, Professor Sekar Kathiresan said that this is the first time that the effect of single-base editing in the liver of non-human primates can be sustained.
On the whole, this means that the dream of reducing LDL once and for all will be realized, and the experimental data makes people very confident that there will be a continuous reduction throughout the life cycle of the animal.
Professor Sekar Kathiresan also stated that toxicology experiments will be carried out in 2021, and IND will be applied for, and the first human clinical trial will be conducted in 2022.
Verve Therapeutics

Verve Therapeutics was founded in 2018 by Professor Sekar Kathiresan and others. It is dedicated to the research and development of gene editing technology to help adults optimize the genome structure and prevent and treat coronary artery disease.
Professor Sekar Kathiresan, a professor at Harvard Medical School, former director of the Center for Genomic Medicine at Massachusetts General Hospital, and director of the Cardiovascular Disease Program of the Broad Institute, is a superstar in the field of heart disease research.
The founder team of Verve Therapeutics is very strong. In addition to Professor Sekar Kathiresan, there are also Professor Kiran Musunuru, a gene editing expert at the University of Pennsylvania, Professor J. Keith Joung, a gene editing expert at Massachusetts General Hospital, and Professor J. Keith Joung or Editas founded by Feng Zhang. Co-founder of Medicine and Beam Therapeutics.
R&D route
In addition to the PCSK9 gene, Professor Sekar Kathiresan also identified six other target genes, all of which can be edited with CRISPR.
These genes will reduce blood lipid levels after being edited, thereby reducing the risk of heart disease, preventing and treating the heart disease.
Before obtaining approval for clinical trials, Verve Therapeutics must also clear many obstacles.
Because statins and other treatments have good results, they want to replace them.
Safety will be the key to obtaining regulatory approval.
Early non-human primate experiments have proved the efficacy and durability of this method.
In addition, the off-target problem faced by single-base editing has always been a major obstacle to clinical application.
The gene editing therapy is based on the single-base editing system of mRNA delivered by lipid nanoparticles (LNP), which is very similar to the delivery strategy of the new coronavirus mRNA vaccine.
Now the success and large-scale production of the new coronavirus mRNA vaccine will greatly reduce LNP The cost of delivery vectors and the cost of mRNA-based therapy will also greatly promote the development of mRNA gene editing components delivered through LNP.
LNP delivers mRNA to lower cholesterol
(sourceinternet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



