Medical Devices Approved by China in 2020
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Medical Devices Approved by China in 2020
Medical devices Approved by China in 2020. As of January 4, 2021, a total of 90 medical devices have been announced to enter the green channel for innovation approval in 2020, of which 37 are Type II and 53 are Type III. Details of the three categories are shown in Table 1.
In 2020, a total of 172 medical devices were announced to enter the green channel for priority approval, of which 161 were in the second category and 11 were in the third category.
In addition, in 2020, a total of 73 medical devices with priority approval of the second category were approved, 5 medical devices with priority approval of the third category were approved, 24 innovative medical devices of the second category were approved, and 26 innovative medical devices of the third category were approved. The details of the three types of innovative medical devices are shown in Table 2.
Table 1 Three types of innovative devices entering the green channel in 2020
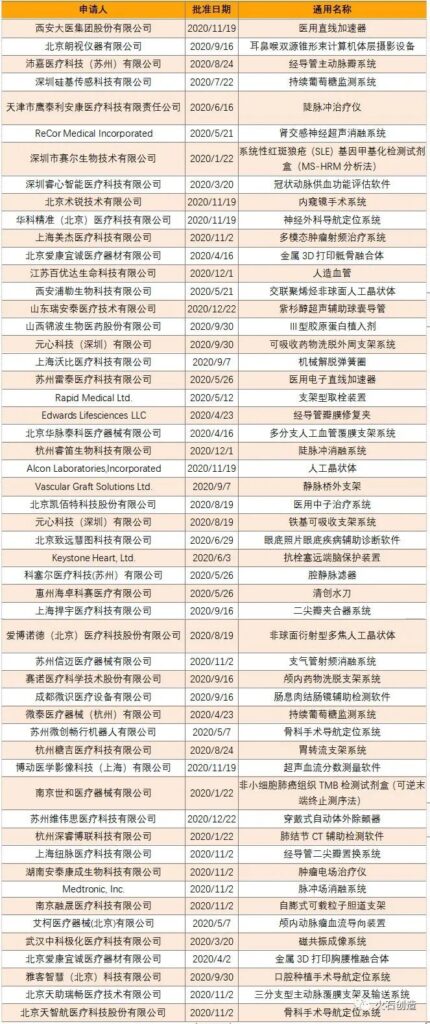
Table 2 Three types of innovative medical devices approved in 2020
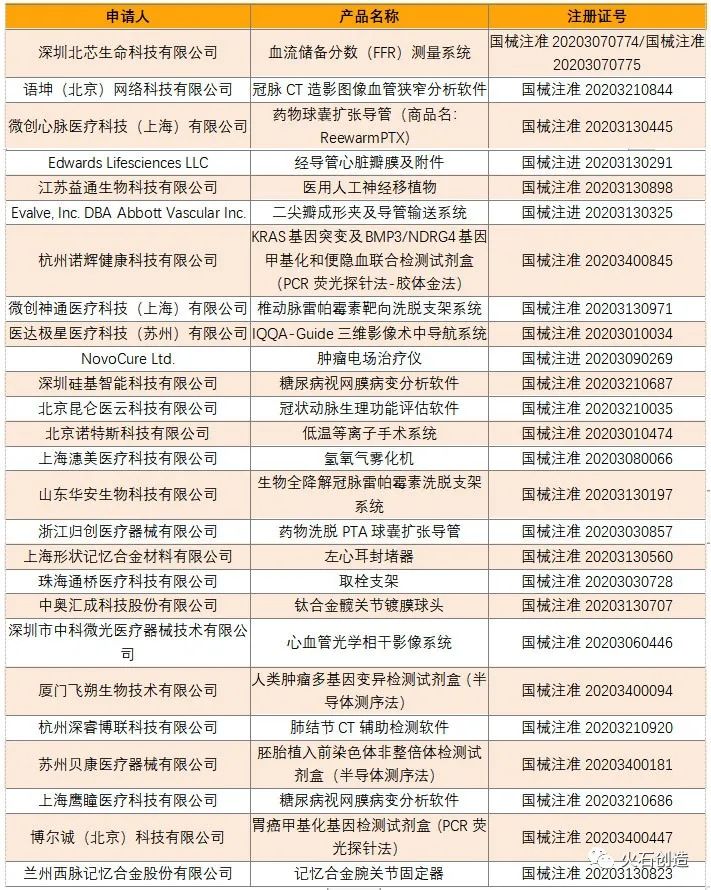
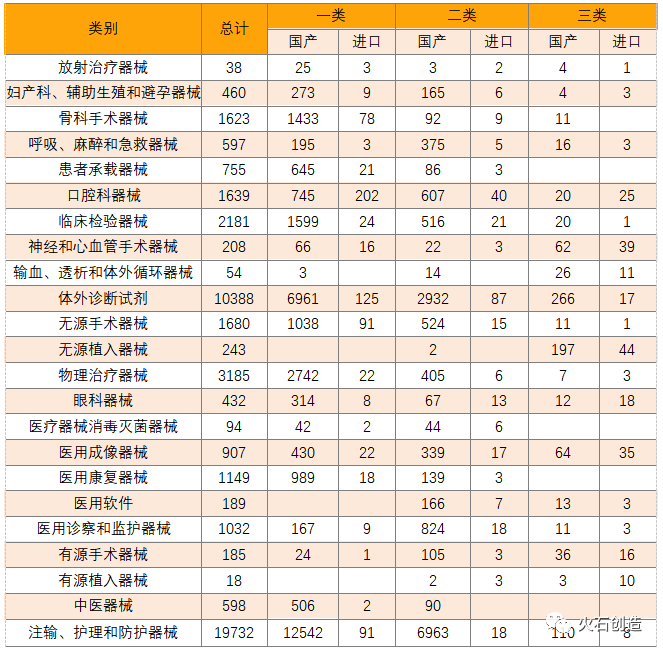
As of January 4, 2021, in 2020, the National Bureau of China has approved a total of 1,134 Class III medical device products, of which 893 are domestically produced and 241 are imported. The provincial drug regulatory authorities have approved domestic Class II medical devices in 2020. 14,482 registered and 30739 first-class medical devices were registered.
Statistics show that affected by the epidemic, the number of infusion, nursing and protective equipment is the largest, a total of 19,732 items, accounting for 41.6%, basically all domestically made, 19,615 items. In addition to infusion, nursing and protective devices, the top three approved registration categories are in vitro diagnostic reagents, physical therapy devices and clinical testing devices.
Table 3 Distribution of the number of approved and registered types of medical devices imported from China in 2020
(sourceinternet, reference only)
Disclaimer of medicaltrend.org



