SINOVAC COVID-19 Vaccine: Why 91% effective in Turkey and 50.38% in Brazil?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
SINOVAC COVID-19 Vaccine: Why 91% effective in Turkey and 50.38% in Brazil?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
China SINOVAC(Kexing) COVID-19 Vaccine: Why 91% effective in Turkey and 50.38% in Brazil.
Butantan Research Institute in Brazil announced the results of the late-stage Kexing(SINOVAC) COVID-19 vaccine. The results showed that the general efficacy of the Kexing(SINOVAC) vaccine was 50.38%.
From bounced tickets to Christmas gifts, and then almost passed on the line at the end, Kexing(SINOVAC) vaccine is currently the one with the most published data among domestic COVID-19 vaccines, and perhaps the most controversial one.
Let’s set the time back to three weeks ago, December 23, 2020.
Data come out very late!
According to the information released by the Butantan Institute in Sao Paulo, Brazil in November, as of November 23, among the 10,800 volunteers participating in the Kexing(SINOVAC) vaccine clinical trial in Brazil, 74 infected persons have been reported, reaching the minimum 61 cases required for effectiveness analysis. The specific data of the vaccine is scheduled to be announced in the first week of December.
By the first week of December, there was no news. Sao Paulo announced that it would announce the news on December 23.
On December 23rd, the Butantan Institute sent a tweet saying that the news will be announced at 4 pm local time on December 23rd .
In the middle of the night, I got up to watch the news and watched the live broadcast.
As a result, Sao Paulo announced that the effective rate of Kexing(SINOVAC)’s vaccine had exceeded 50%, but due to Kexing(SINOVAC)’s requirements, the release of specific vaccine data would be delayed for another 15 days.
A day later, Turkey, which is half the world apart from Brazil, announced the interim results of the Phase III clinical trial of Kexing vaccine on Christmas Eve: an effective rate of 91.25%.
Everyone can’t remember the pigeons released by Brazil the day before, and the mood at this moment is extremely heroic.
This is the most efficient inactivated vaccine so far. Even compared with previous mRNA vaccines, this data is not much different.
Not only is the effective rate announced, Turkey also announced many other details of the Kexing(SINOVAC) vaccine clinical trial.
The Phase III clinical trial of Kexing Vaccine in Turkey started on September 14, 2020, and it plans to recruit 13,000 volunteers.
Like Sinopharm vaccine, Kexing vaccine is also an inactivated vaccine, requiring two injections, 14 days apart.
The primary endpoint of clinical trials is the proportion of infected persons with symptoms, which is also the most important indicator of protection, and is much more important than measuring the neutralizing antibody gradient in phase I/II.
As of Christmas, more than 7,000 people have been enrolled in clinical trials in Turkey, and the results announced this time are based on 1,322 of them.
Twenty-nine out of 1,322 people were infected, of which 26 were in the placebo group and 3 were in the vaccine group.
- There were 752 people in the vaccine group, 3 people were infected, of which 1 was mild and 2 were asymptomatic, and the infection rate was 0.399%
- 570 people in the placebo group, 26 people were infected, 6 of which were severely ill, the infection rate was 4.561%
- Effective rate=(4.561-0.399)/4.561=91.25% (95% confidence interval: 71.3-97.3%)
- No major security incidents seen
Compared with the 86% effective rate of the national medicine vaccine announced by the UAE, Turkey’s data this time has more important details (the number of volunteers and the number of infections in the two groups), which is a great improvement.
However, compared with the results published by several other COVID-19 vaccines (Pfizer vaccine, Moderna vaccine and Oxford vaccine), Kexing(SINOVAC)’s data dimensions are still a little less, such as the types of adverse reactions and the proportions that occur in different groups, and the infected persons The proportion of severe cases in the population.
Why is the data of only 1,322 released among 7,371 people?
Because the third phase of Turkey is divided into two separate cohorts, K1 is a high-risk group, including medical staff such as doctors, nurses, cleaners, and hospital administrators; K2 is a normal-risk group. This time 1322 people are all K1.
Some people may worry: Will high-risk groups affect efficiency?
The answer is no, because high-risk groups are both in the placebo group and the vaccine group. High-risk accelerates the infection rate of the two groups, and the effective rate depends only on the difference in the infection rate between the two groups.
The K1 data is released first, which helps to promote the current vaccine among high-risk groups such as medical staff, and there is nothing wrong with it.
However, when entering 2021, Kexing Vaccine released the most important data, and it also ushered in huge public opinion.
On January 7, the Butantan Institute in Brazil finally announced the results of the late-stage clinical trial of the Kexing(SINOVAC) Vaccine-an effective rate of 78%.

Source: Butantin Institute Twitter
- Although there is a gap between this result and Turkey’s, this is still one of the most important results of all China’s COVID-19 vaccines, because the clinical trial of Kexing(SINOVAC) in Brazil covers the two types of people most in need of protection-the elderly and patients with underlying diseases .
- This is what some other vaccine clinical trials lack.
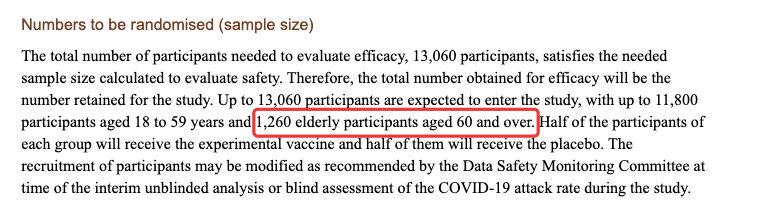
Source: Reference 1
The entry requirements of Kexing’s clinical trials in Brazil are not high. 1. The entry age is ≥18 years old, and there is no upper limit; 2.
Some volunteers with underlying diseases can be enrolled. The exclusion criteria in clinical trials in Brazil are mainly basic diseases and AIDS that cannot be controlled after treatment.
Based on the coverage of more high-risk groups, 78% of the effective rate is not the past.
But almost beyond everyone’s expectations, this 78% is not the final figure.
In the early morning of January 12, Sao Paulo, Brazil held a press conference to announce more details. The most important point is that the overall effective rate of Kexing vaccine is 50.38%.
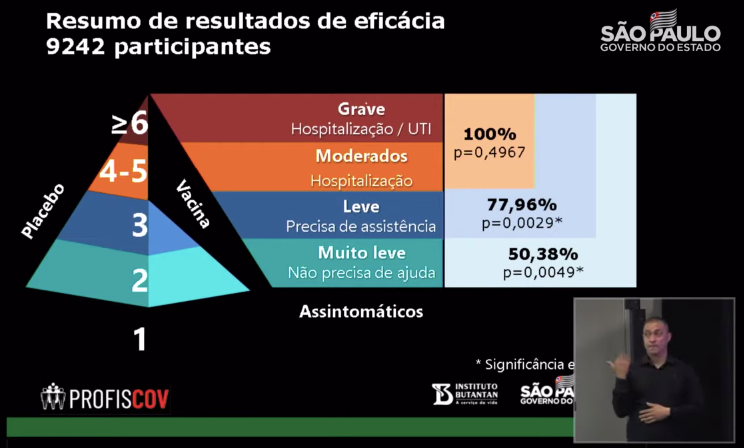
Source: Official YouTube of the State of Sao Paulo, Brazil
Why is it also in Brazil that 78% was announced a few days ago, but today it is 50.38%?
According to the information released at the press conference, Brazil classified the symptoms of new coronavirus cases in clinical trials into 6 levels, namely asymptomatic, mild, mild, and two levels of moderate and severe. The first two levels do not require medical intervention. .
- According to Brazil, 78% belongs to clinical efficacy, which refers to the probability of preventing mild disease (mild); and the 50.38% announced this time is the overall effective rate, including the probability of preventing mild disease and mild symptoms.
- To put it simply, after taking the Kexing vaccine, it can reduce the chance of any new coronavirus pneumonia-related symptoms by 50.38%, and at the same time, there is a 78% chance that there will not be any new coronavirus pneumonia-related mild or severe illness that requires medical intervention.
Also, as previously announced, it can reduce your 100% probability of developing severe illness. The phrase “100% avoid severe illness” is also included in the headline of many domestic media reports.
But for this point, there is a certain controversy for the time being: because there are too few data. Seven severe cases were found in the placebo group, with a p-value of 0.49, which indicates no significant difference.
How is the 50.38% effective rate calculated?
Regarding this final effective result, there is another point that many people are concerned about: how is the 50.38% effective rate calculated?
According to the information disclosed at the press conference, there were 4653 volunteers in the vaccine group and 85 people infected; 4,599 people in the placebo group, 167 people infected.
The infection rate of the vaccine group was 1.83%, and the infection rate of the placebo group was 3.63%, which resulted in a population protection of 50.38% (p=0.0049).
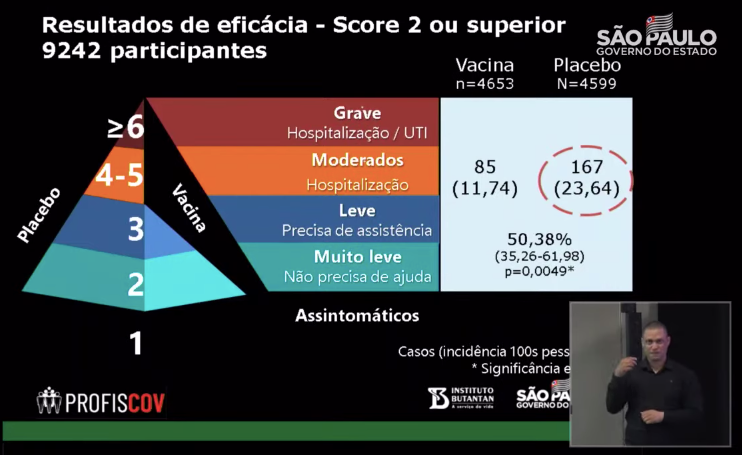
Source: Official YouTube of the State of Sao Paulo, Brazil
According to the “Principles of Epidemiology in Public Health Practice, Third Edition” on the official website of the CDC of the United States, Vaccine efficacy/effectiveness (VE) represents The proportion of disease reduction in the group.
Its calculation formula = (placebo group (or group not receiving vaccine) risk-vaccine group risk) / placebo group infection rate.
According to this consensus, the effective rate demonstrated by Kexing in Brazil’s clinical trials should be (167/4599-85/4653) ÷ (167/4599)=49.69%.
Although the difference between 49.69% and 50.38% is small, it is very subtle and directly determines whether the effectiveness of this vaccine falls below the 50% red line recommended by WHO.
Regarding this issue, we still have to look back at the clinical trial protocol.
In statistics, the method that also considers the variable risk exposure time is called survival analysis (that is, the method of studying the relationship between influencing factors and survival time and outcome), and its main measurement index is the risk rate (HR, Hazard Rate).
In statistics, there is also a classic analysis method that only considers the number of infections in each group, without considering the exposure time. It is measured by odds ratio (OR) or relative risk (RR).
In the Phase III clinical trial of Kexing(SINOVAC) Vaccine in Brazil, the primary objectives are to assess the virus infection of symptomatic individuals 2 weeks after receiving two shots of Kexing(SINOVAC) vaccine.
The efficient method used by the Butantan Institute in Brazil is the hazard ratio.
Simply put, compared to the method we mentioned above, the hazard ratio will also add the factor of time.
The hazard ratio calculates the cumulative incidence rate per unit time, and the result of (1-hazard ratio) is that this method calculates effectiveness.
In the effectiveness analysis of the hazard ratio, the researchers added up the exposure time of each volunteer after vaccination to calculate the infection rate based on time.
There is no distinction between various statistical methods, and the choice of which one to use depends on the design and purpose of the research.
The most important thing is that the method is clear before the start of the clinical trial so that the results will not deviate from the initially set goals.
If the observation time of clinical trials is extended enough, for example, two years, the result calculated by the hazard ratio and the result calculated by our commonly used formula will tend to be consistent; but if the observation time is short, there may be a gap between the two results There are certain differences.
According to the calculations of Butantan researchers, the risk of infection in the vaccinated group was 11.74 and that in the placebo group was 23.64.
Therefore, the effective rate of Kexing vaccine was calculated as (23.64-11.74)/23.65 = 50.34%.
Since this (referring to the Phase III early study of Kellyford) is a short-term study, the exposure time of each volunteer to the risk of infection may be very different, so the data of the Kellyford study chose to consider the risk of exposure at the same time Time hazard ratio calculation.
It is reported that the plan was submitted to the National Health Surveillance Agency (Anvisa) in August to obtain the approval of the research and was published in a peer-reviewed scientific journal.
Waiting for more data to be released
From the 91% announced by Turkey at the end of December, to the 78% announced by Brazil last Thursday, to the 65% announced by Indonesia the day before yesterday, to the final 50.38%, it is reasonable that this change will attract some attention.
Similarly, there are huge differences in Phase III clinical trials of Kexing vaccine in different countries (Turkey 91%, Indonesia 65%, Brazil 50.38%), which one is more worthy of reference for Chinese people?
No one knows, maybe we need more data.
Looking at the long history, many vaccines have been marketed with controversy. These vaccines have ultimately helped us control or even eliminate certain infectious diseases.
This COVID-19 vaccine is no exception.
From Oxford vaccine to Pfizer vaccine, from Sinopharm vaccine to Kexing vaccine, the controversy is huge. It has a lot to do with the severely shortened development time of the COVID-19 vaccine itself, and because they carry too much hope for mankind.
On the same day that Brazil released the data, on the morning of January 13, the 59-year-old Indonesian President Joko Widodo was vaccinated with Koxing. Indonesian people witnessed the entire process of the president’s vaccination through live broadcast.
SINOVAC COVID-19 Vaccine: Why 91% effective in Turkey and 50.38% in Brazil
SINOVAC COVID-19 Vaccine: Why 91% effective in Turkey and 50.38% in Brazil
SINOVAC COVID-19 Vaccine: Why 91% effective in Turkey and 50.38% in Brazil
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



