Biacore plays important role in COVID-19 vaccine development
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Biacore plays important role in COVID-19 vaccine development
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Biacore plays important role in COVID-19 vaccine development.
As the “gold standard” of molecular interaction, since the outbreak of the COVID-19 epidemic, Biacore has been involved in the basic research of virus invasion mechanism, viral protein structure, and the development of new coronavirus therapeutic drugs, including the screening, characterization and competition of antibody drugs and small molecule drugs. Played an important role in the research.
In the development of the COVID-19 vaccine, Biacore has also made outstanding contributions. Many COVID-19 vaccines that have been on the market or under development have used Biacore in different stages of development. Below, the editor will give you a detailed introduction to the main applications of Biacore in the development of different types of vaccines.
Nucleic acid vaccine
The nucleic acid vaccine delivers the gene encoding the antigen into the human body, and then uses the host’s processing plant to produce the antigen protein after entering the cell, so that the corresponding antibody is produced in the body, thereby inhibiting virus infection. Among them, the mRNA vaccine introduces mRNA encoding the antigen. Compared with traditional vaccines, there is no need to obtain virus strains, and the vaccine production does not require cell culture. The production process is simple and has significant advantages.
The mRNA vaccine BNT162b2, jointly developed by BioNTech and Pfizer, is the world’s first COVID-19 vaccine approved for marketing. BNT162b2 encodes the new coronavirus spike protein, which is a nucleoside modified and codon-optimized mRNA vaccine.
In the study of this vaccine, Biacore was also used. In the published preclinical data of the BNT162b2 vaccine, the researchers used the advantage of Biacore to directly detect complex samples to detect the binding ability of neutralizing antibodies and S protein-related antigens in vaccinated animal serum samples.
In this study, the researchers used the anti-mouse-Fc antibody coupled to the chip to directly capture the neutralizing antibody produced in the serum of the immunized mouse on the surface of the chip, and then detected the interaction with the new coronavirus S1 subunit and RBD respectively. Interaction (Figure 1). The results showed that BNT162b2 successfully produced neutralizing antibodies that specifically recognized the virus in mice.
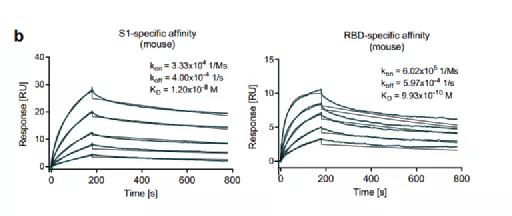 Figure 1 Binding of antibodies in the serum of immunized mice to the S1 subunit of the virus and RBD
Figure 1 Binding of antibodies in the serum of immunized mice to the S1 subunit of the virus and RBD
Soon after the release of this research result, in September 2020, BioNtech once again disclosed the research and development data of BANCOVID, an mRNA vaccine developed by it using the mutated new coronavirus D614G strain.
As we all know, virus mutation has always been a thorny issue. New mutant strains of the new coronavirus may not only make the new coronavirus more capable of spreading, but also allow the virus to evade recognition by the immune system, which means that these mutant viruses may be Escape the protection of existing vaccines.
In July 2020, the World Health Organization announced that 29% of the COVID-19 virus samples have the D614G mutation, and this mutation may enhance the spread of the COVID-19 virus. BioNtech’s BANCOVID vaccine is an mRNA vaccine developed for this mutation.
In the development of the BANCOVID vaccine, competitive experimental data represented by Biacore showed that specific antibodies against the entire extracellular domain (S1 and S2 subunits) of the S protein were produced after vaccination.
In this experiment, the researchers coupled the S1 and S2 subunits of the Sipke protein of the new coronavirus to the chip at the same time. After the immunized mouse serum was injected, it was significantly bound to the fixed S1+S2 protein, proving that the immune system had targeted S1/S2 antibody.
Pre-mixing S1 or S2 or S protein in mouse serum all reduced the response value to varying degrees, proving that the antibodies produced bind to both S1 and S2 antigens (Figure 2, left). After purifying the antibody in the immunized mouse serum, the Biacore competition experiment was repeated, and the same result was obtained (Figure 2, right).
This indicates that BANCOVID vaccine produced neutralizing antibodies against the entire Spike protein after immunizing mice. Combined with the results of other animal experiments, it shows that BANCOVID is a good vaccine candidate against the new coronavirus D614G strain.
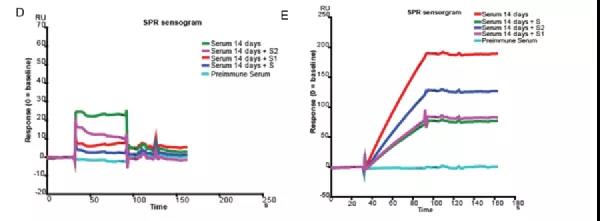
Figure 2 Biacore detects the binding of antibodies in the serum of immunized mice to the virus S protein
Subunit vaccine
Compared with traditional inactivated or attenuated live vaccines, subunit vaccines do not use the whole virus but select the part of the virus that can stimulate the human body to produce immunity to imitate the whole virus.
A large number of basic studies have shown that the key step in the process of new coronavirus infection is to use the receptor binding domain (RBD) of the virus surface S protein to interact with the host cell receptor angiotensin converting enzyme (ACE2) to infect host cells.
The S protein has always been a golden target for vaccine development, and most of the subunit vaccines of the new coronavirus are developed based on part of the virus S protein. Below we will sort out several reported subunit recombinant protein vaccines.
On July 29, 2020, Nature magazine published the first COVID-19 vaccine research paper, which came from the State Key Laboratory of Biotherapy, West China Hospital, Sichuan University. It reported the RBD of SARS-CoV-2 Spike protein expressed in insect cell culture. Residues 319-545 are the research results of candidate vaccines.
After a single dose of the recombinant protein vaccine immunized mice, rabbits and non-human primates, it produced effective functional antibody responses on the 7th or 14th day, and neutralized the SARS-CoV-2 pseudovirus and live virus in vitro Infection, provides protection against SARS-CoV-2 in non-human primates. The vaccine was approved by the State Food and Drug Administration on August 21.
In this study, the researchers verified the binding of recombinant RBD protein to ACE2 through Biacore. The results showed that RBD recognized ACE2 with an affinity of 1.54 nM (Figure 3), indicating that the recombinant RBD protein expressed by insect cells has a natural conformation and retains Immunogenicity provides a basis for the development of protective vaccines.
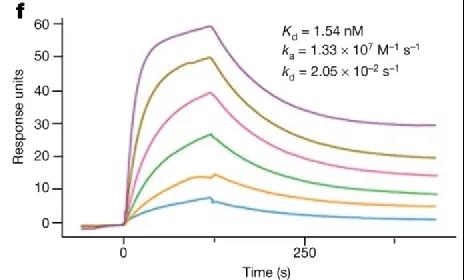
Figure 3 Interaction between recombinant RBD protein and ACE2 receptor
The COVID-19 recombinant protein subunit vaccine developed by the Gao Fu team of the Institute of Microbiology of the Chinese Academy of Sciences in conjunction with Anhui Zhifeilongkoma has completed Phase 1 and Phase 2 clinical trials, and is conducting international multi-center Phase 3 clinical trials.
The researchers considered the limited immunogenicity of the RBD domain of the coronavirus S protein, and the vaccine designed to induce the body to produce neutralizing antibodies is poor. Based on this, the new coronavirus S protein RBD dimer subunit vaccine was designed, and experiments have proved that it has better immunogenicity and protection than RBD monomer.
So is the constructed RBD dimer the same as the monomer, retaining the original receptor binding domain (RBM)? Through Biacore experiments, it was found that the designed RBD dimer with a stable tandem repeat single chain, RBD-sc-dimer, has the same affinity as the ACE2 receptor compared with RBD monomer (Figure 4). The binding crystal structure showed that the dimerized RBD completely exposed the dual receptor binding domain, which is also the main binding site of the neutralizing antibody, so this dimerized RBD successfully retained the vaccine’s efficacy.
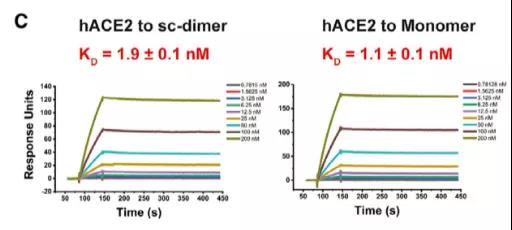
Figure 4 Comparison of affinity between RBD-sc-dimer and RBD monomer and ACE2 receptor
It is worth mentioning that this vaccine design strategy is also applicable in the development of vaccines against MERS and SARS.
The researchers also used Biacore to detect the binding of the dimer antigens of MERS and SARS to the corresponding receptors, and the results showed that the affinity of the dimer antigens of MERS and SARS to the corresponding receptors was comparable to that of monomers (Figure 5), and made The titers of neutralizing antibodies produced by immunized mice increased significantly.
It shows that this strategy can be generally applied to the vaccine design of other β-coronaviruses, so as to deal with the new infectious diseases caused by the coronavirus that may appear in the future.
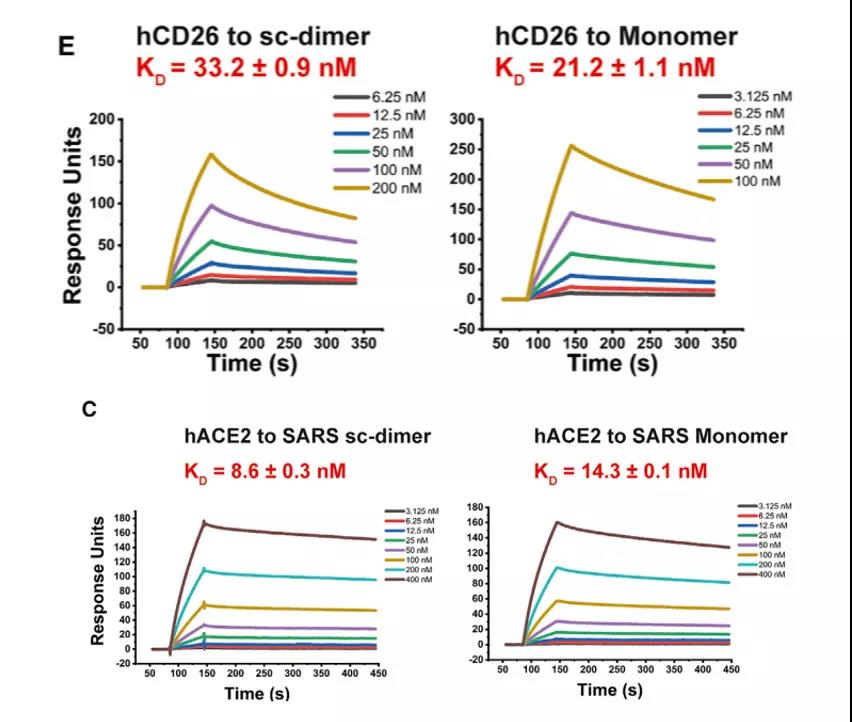
Figure 5 Comparison of the affinity of dimerized RBD and monomer RBD of MERS and SARS viruses with corresponding receptors
It is also a recombinant subunit vaccine. In December 2020, Xia Ningshao’s team at Xiamen University published a research result on a new coronavirus recombinant subunit vaccine on the preprint website bioRxiv. This subunit vaccine is a virus Sipke cell expressed by CHO cells.
The composition of exoprotein (StriFK) and modified zinc-aluminum adjuvant (FH002C) can quickly trigger strong humoral and cellular immune responses in animal models.
The researchers used Biacore to detect the binding of the StriFK protein to the ACE2 receptor (Figure 6), confirming that the expressed StriFK protein retains the ability to bind ACE2, which provides a solid foundation for the further optimization of the vaccine and clinical trials.
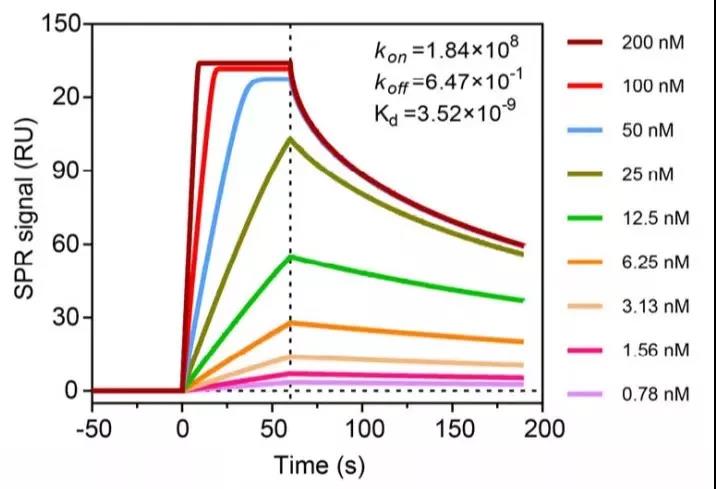
Figure 6 The affinity of the new coronavirus Spike extracellular protein StriFK with the ACE receptor
Nanoparticle vaccine
Similar to subunit vaccines, nanoparticle vaccines are also designed by selecting key viral proteins as antigens. But the difference is that the nanoparticle vaccine connects the antigen to the nanoparticle, and uses the self-assembly of the nanoparticle to enrich the effective antigen, realizes a multi-antigen recombinant protein vaccine, and greatly improves the immunogenicity of the vaccine.
The nanoparticle vaccine developed by Professor Zhang Hui’s research group from the Institute of Human Virology, Sun Yat-sen University can display 24 identical or different new coronavirus antigens on the surface of a single vaccine at the same time, enhancing the vaccine’s protective immune response, and the induced neutralizing antibodies can be Significantly resist the body from being infected by the new coronavirus.
The researchers constructed two ferritin nanoparticle vaccines (Figure 7), one is RBD nanoparticle vaccine, and the other is RBD-HR (heptad repeat) chimeric nanoparticle vaccine.
Through Biacore interaction experiments, it was found that the two nanoparticle vaccines constructed have the same ability to bind to the ACE2 receptor as the RBD monomer, and have the same affinity (Figure 7), which lays the foundation for further optimization of subsequent vaccines.
The article claims that the neutralizing antibody titer against the new coronavirus induced by this nanoparticle vaccine in mice ranks first in the world and can be used as a potential new coronavirus vaccine.
The COVID-19 nanoparticle vaccine is currently applying for clinical approval to the State Food and Drug Administration.
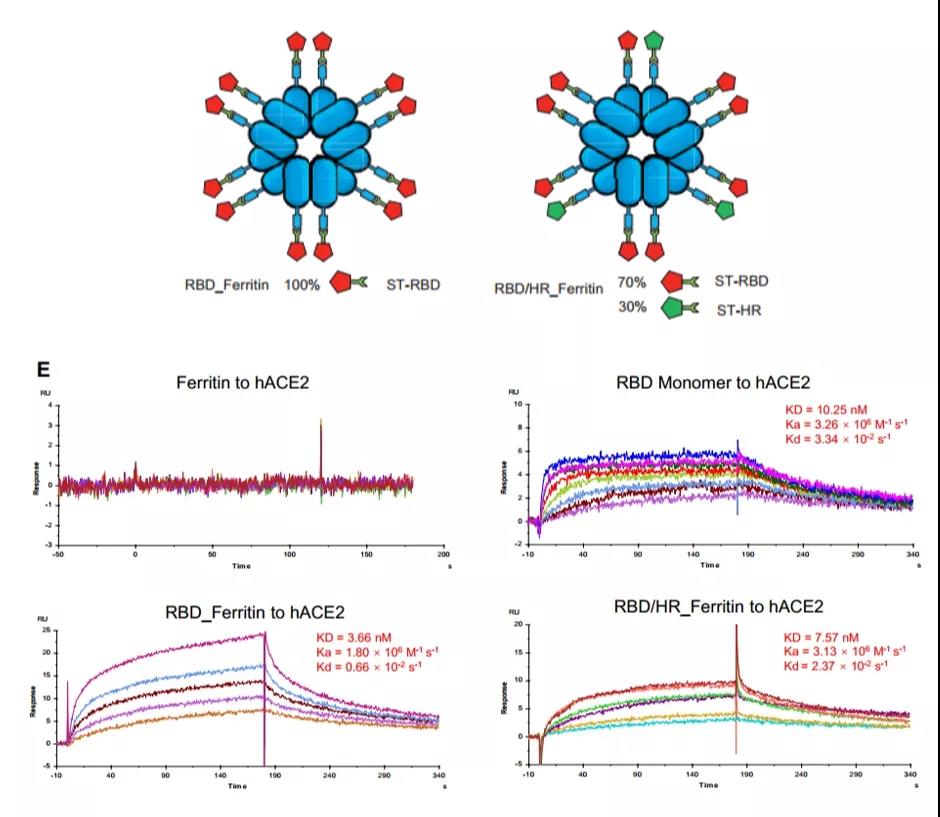
Figure 7 RBD monomer and two nanoparticle vaccines constructed
Combination of RBD_Ferritin and RBD/HR_Ferritin with ACE2
Based on the above research results, we can see that Biacore is widely used in nucleic acid vaccines, subunit vaccines and nanoparticle vaccines.
It can not only detect the binding between purified proteins, but also directly detect complex samples such as serum, which is very important in the development of vaccines and can greatly improve the efficiency of experiments.
The reason why Biacore can detect complex samples is because of its extremely high sensitivity, ultra-low non-specific adsorption, and rigorous background subtraction methods to eliminate the influence of impurities on the experiment.
From the perspective of application, Biacore can not only be used to characterize the binding affinity between vaccine antigens and receptors or antibodies, but also can easily design competition experiments to intuitively discover antibody binding epitopes, thanks to Biacore’s pipeline design and Continuous injection mode.
And for antigen-binding epitopes, Biacore also has a professional epitope module to help researchers quickly process large amounts of data and present various results graphically.
In addition to the development of the COVID-19 vaccine, Biacore can also be seen in the development and quality control of a variety of vaccines in the past, including influenza vaccine, HPV vaccine, herpes vaccine and IPV vaccine. In this battle against the epidemic, Biacore also used precise data and diverse applications to escort the development of a variety of COVID-19 vaccines.
In the new year, we will continue to invest and work together with the majority of scientific researchers to enable more and more COVID-19 vaccines to move from research and development to the market, so that more people can use safe, effective and accessible vaccines. .
Biacore plays important role in COVID-19 vaccine development
(source:internet, reference only)
Disclaimer of medicaltrend.org



