Phloridin improves obesity-related endotoxemia and insulin resistance
Phloridin (PHZ) improves obesity-related endotoxemia and insulin resistance
Phloridin (PHZ) improves obesity-related endotoxemia and insulin resistance. Gut Microbes: Phloridin improves obesity-related endotoxemia and insulin resistance by interfering with the intestinal flora and intestinal barrier integrity of obese mice.
More and more evidences show that plant-derived polyphenols (including flavonoids, phenolic acids, hydroxyphenols and catechins) can improve the antioxidant and anti-inflammatory status of the intestinal tract, thereby enhancing the epithelial barrier function and regulating the intestinal tract The flora effectively prevents and treats metabolic syndrome.
Guide
Phloxidin (PHZ) is a phytonutrient in apples, which helps promote health. As the saying goes, “An apple a day keeps the doctor away.” Previous studies have shown that phlorizin can competitively inhibit the reabsorption of glucose by SGLTs and thus have efficacy in the treatment of diabetes. However, the extremely low bioavailability of phlorizin hinders its development and application. In this paper, high-throughput sequencing technology and fecal bacteria transplantation are used to study the improvement of obesity induced by high-fat diet in mouse models by adjusting the “gut microbe-barrier axis”. The author revealed that phloridin has a high-efficiency therapeutic effect in animal experiments despite its low bioavailability. This study provides an important basis for the application of phloridin as a new prebiotic in the prevention and treatment of metabolic syndrome.
Paper ID
- Original name: Phlorizin ameliorates obesity-associated endotoxemiaand insulin resistance in high-fat diet-fed mice by targeting the gutmicrobiota and intestinal barrier integrity
- Journal: Gut Microbes
- IF: 7.740
- Posting time: 2020.11
- Corresponding author: Zhang Xiao Yu
- Corresponding Author Unit: College of Life Sciences, Sichuan Normal University
Experimental design
In the experiment, mice were divided into four groups (n = 10 in each group): (1) Ordinary diet group (fed ordinary diet, 10% of calories come from fat; Supplementary Material Table S1); (2) Ordinary diet plus phlorizin Group (fed with NCD and 80 mg/kg intragastric PHZ); (3) high-fat diet group (fed with high-fat diet, 60% of calories come from fat; supplementary material table S1); (4) high-fat diet with phloretin Group (fed HFD and 80 mg/kg intragastric PHZ).
The experiment lasted for 12 weeks, and the weight and food intake of the mice were measured every week. During the first four weeks of the fecal bacteria transplantation experiment, the feces of four groups of mice (as donor mice) were collected daily for further analysis. Stool samples were collected and stored at -80°C until use.
In the 12th week, glucose tolerance test and insulin tolerance test were performed with 2.0 g/kg glucose and 0.75 U/kg insulin, respectively. After fasting for 12 hours, all mice were sacrificed. Serum samples were collected by centrifugation at 1700 rpm for 10 minutes, and the supernatant was stored at -80°C. The experimental design is shown in the figure.
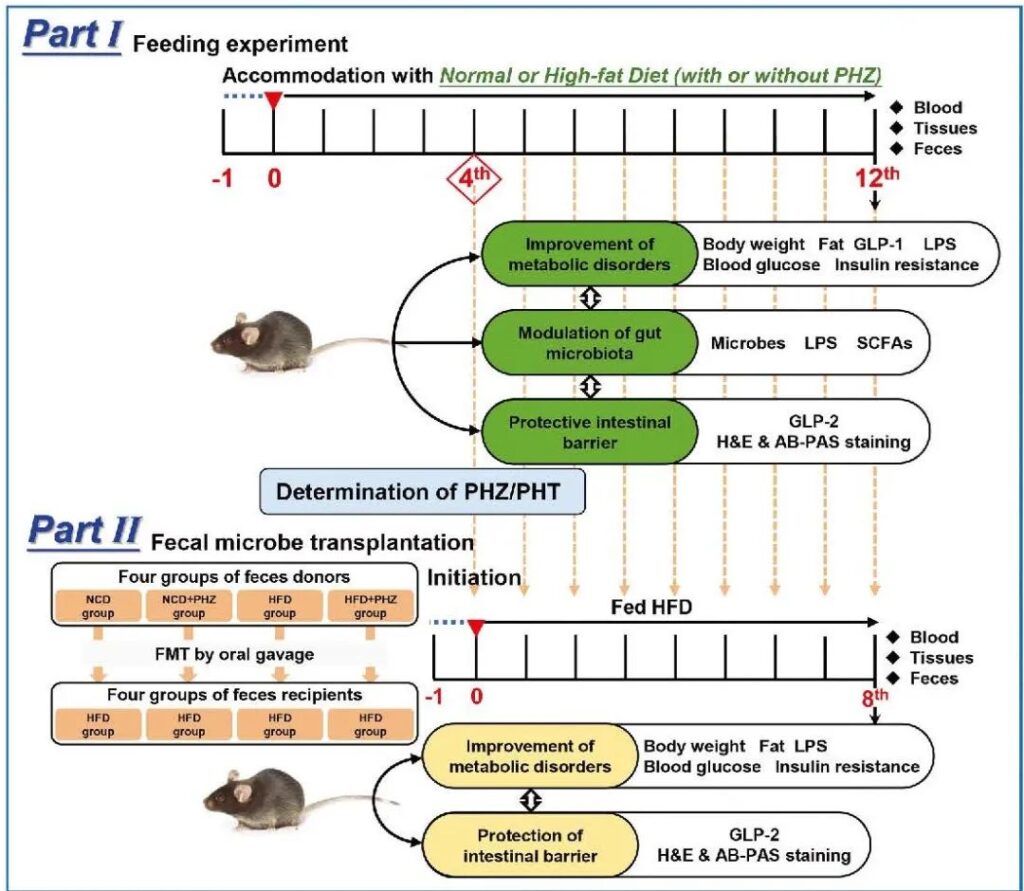
Experimental design. PHZ: Phloridin; PHT: adrenocorticotropic hormone; GLP: glucagon-like peptide; LPS: endotoxin; SCFA: short-chain fatty acid; H&E: hematoxylin and eosin; AB-PAS: periodic acid mat Husband; NCD: normal feed; HFD: high-fat feed. FMT: Fecal flora transplantation.
Results:
1 Phloridin inhibits obesity-related symptoms induced by high-fat diet
C57BL/6 J mice fed a normal diet or a high-fat diet were co-administered with or without phlorizin (80 mg/kg) for 12 weeks. The results showed that co-administration of phlorizin can effectively inhibit weight gain caused by high-fat diet, and significantly reduce body weight from the 3rd week (P <0.001; Figure 1a).
However, there were no significant differences in food intake (Figure 1b) or energy intake (Figure 1c) between the ordinary diet and the ordinary diet plus phlorizin group or between the high-fat diet and the high-fat diet plus phlorizin group (Figure 1c). In this case, P>0.05), indicating that the anti-obesity effect caused by the co-use of phlorizin is not due to the reduction of food consumption or energy extraction. The reduction in fat deposits (Figure 1d) and the weight changes of different organs (Figure 1e) further confirm this.
The morphological results showed that phlorizin significantly inhibited fat deposition in the liver induced by high-fat diet (Figure 1f), which may effectively reduce the further development of complex diseases such as fatty liver. On the other hand, phlorizin significantly inhibited fat deposition induced by high-fat diet (Figure 1g) and fat cell enlargement (Figure 1h, i, P <0.001).
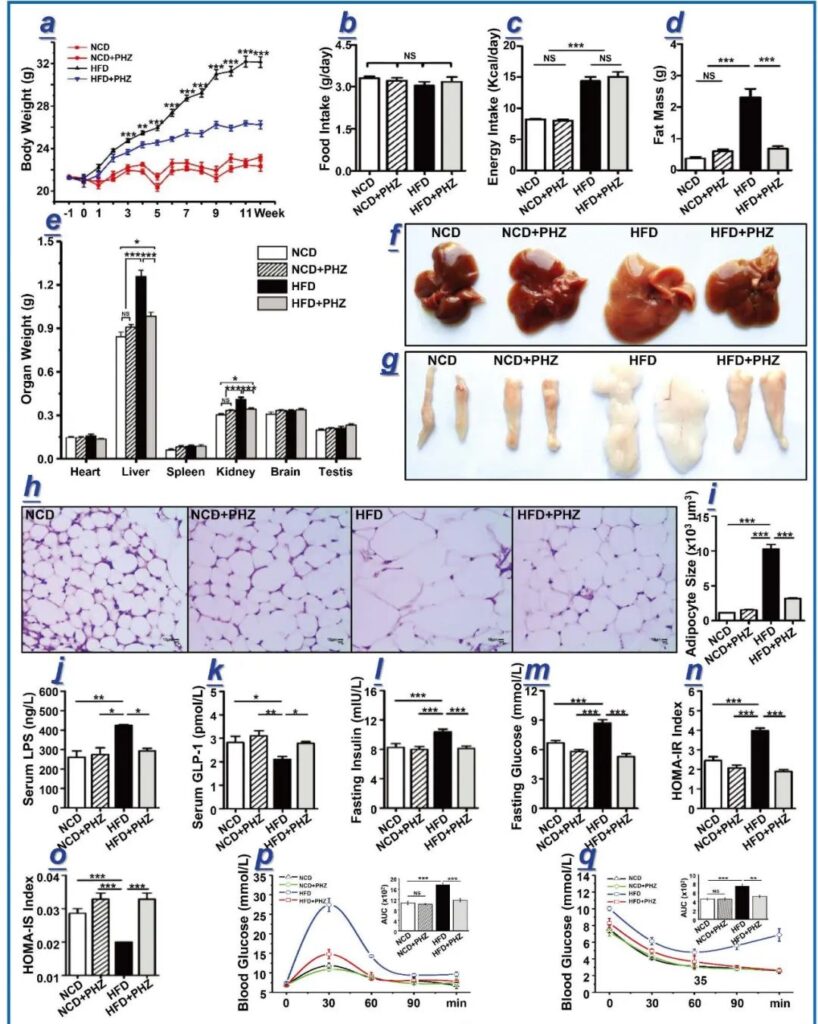
Figure 1 Phlorizin relieves obesity caused by high-fat diet (HFD). (A) The weight change of mice at 12 weeks. In the 12th week, count the four groups of mice [normal diet (NCD), normal diet with phlorizin (NCD + PHZ), high-fat diet (HFD) and high-fat diet with phlorin (HFD + PHZ)] and Obesity-related parameters. Parameters include: (b) food intake; (c) energy absorption; (d) fat weight; (e) weight of different organs; (f) liver and (g) morphological observation of epididymal fat; (h) Hematoxylin and eosin staining of epididymal fat; (i) fat cell size of epididymal fat; serum (j) lipopolysaccharide (LPS) and (k) glucagon-like peptide 1 (GLP-1) levels; (L) Insulin in fasting blood; (m) Fasting blood glucose; (n) Homeostasis model assessment (HOMA)-insulin resistance (IR) index; (o) HOMA-insulin sensitivity (IS) index; (p) oral Glucose tolerance test; (q) insulin resistance test. The data are expressed as mean ± standard deviation. One-way analysis of variance was used to analyze statistical differences; P> 0.05, * P <0.05, ** P <0.01 and *** P <0.001.
As a component of the cell wall of gram-negative bacteria, LPS is considered to be a trigger factor that promotes the occurrence and development of systemic inflammation and related metabolic syndrome through a variety of ways. In addition, the increase in LPS induced by a high-fat diet can lead to endotoxemia. Similarly, this study found that the serum LPS level of mice fed a high-fat diet was significantly increased (P <0.01), and the addition of phlorizin significantly inhibited the increase of serum LPS (P <0.05; Figure 1j).
Long-term systemic inflammation has also been shown to trigger glucose tolerance and insulin resistance, which are closely related to the onset and development of obesity. As one of the most important intestinal hormones, glucagon-like peptide 1 (GLP-1) stimulates glucose-dependent insulin secretion, thereby improving metabolic syndrome, such as obesity and type 2 diabetes. In this study, the high-fat diet significantly reduced the serum GLP-1 level (compared with the normal diet group, P <0.05; compared with the normal diet plus phlorizin group, P <0.01); Addition significantly inhibited this effect (P <0.05; Figure 1k).
As a result, the elevated levels of fasting blood insulin (Figure 1l), glucose (Figure 1m) and HOMA-IR (Figure 1n) induced by a high-fat diet were significantly prevented (P <0.001 in all cases). On the other hand, a high-fat diet significantly suppressed the level of HOMA-IS, but the addition of phlorizin prevented this effect (P <0.001; Figure 1o). In addition, compared with the high-fat diet group, the area under the curve (AUC) of the mice in the high-fat diet plus phlorizin group in the oral glucose tolerance test (OGTT; Figure 1p) and insulin tolerance test (ITT; Figure 1q) The value is significantly reduced (P <0.001 and P <0.01, respectively).
2 Phloridin alleviates intestinal microbial disorders and metabolic malnutrition induced by high-fat diet
Previous studies have shown that phloridin directly reduces blood sugar levels by competitively inhibiting SGLT2, and plays a key role in the prevention and treatment of metabolic syndrome. However, the extremely low bioavailability of phlorizin makes people wonder whether this is the only mechanism behind the therapeutic effect. Therefore, the current work is to hypothesize that the intestinal flora is an alternative target that leads to the beneficial effects of phlorizin intake. This work applied high-throughput sequencing technology to systematically analyze the changes in fecal microbiota composition after phloridin supplementation.
First, a principal component analysis (PCA) was performed to visualize the differences in the structure of the fecal microbiota between the four groups (Figure 2a; PC1 and PC2 were 41.5% and 10.1%, respectively). Different clustering patterns based on groups are observed in the score map. The symbols representing obese mice induced by high-fat diet are all distributed in the right quadrant, while the symbols representing most mice in the other three groups are located in the left quadrant, indicating that the structure of the fecal flora of obese mice induced by high-fat diet and the other three groups exists A huge difference.
Although they showed a unique aggregation pattern in the lower left quadrant of the high-fat diet plus phlorizin group, they clustered closely with the normal diet and ordinary diet plus phlorizin group (mainly distributed in the upper left quadrant on the score chart). ), indicating that the three groups share higher similarities in the structure of the intestinal flora, although there are still significant differences. Together, these results indicate that a high-fat diet significantly changes the structure of the intestinal flora.
However, the joint use of phloridin can effectively inhibit or alleviate the changes in the intestinal flora. The symbols representing ordinary diet and ordinary diet plus phlorizin mice are mainly distributed in the upper left quadrant, appearing in the form of two close clusters, indicating that there are still significant differences in the structure of the intestinal flora between the two groups. These results further confirm that phlorizin supplementation can specifically affect the intestinal flora. The results of PCA are basically consistent with the results of hierarchical clustering analysis (Figure 2b), except that sample 2-1 does not cluster with any group on the PCA score map.
Interestingly, based on the PCA results, there are significant differences in the structure of the intestinal flora between the groups, and the high-fat diet did completely change the structure of the intestinal flora, but the alpha diversity between the groups (expressed by the Chao1 index) No significant difference was detected (Figure 2c, P> 0.05), indicating that different interventions did not cause significant changes in taxa richness.
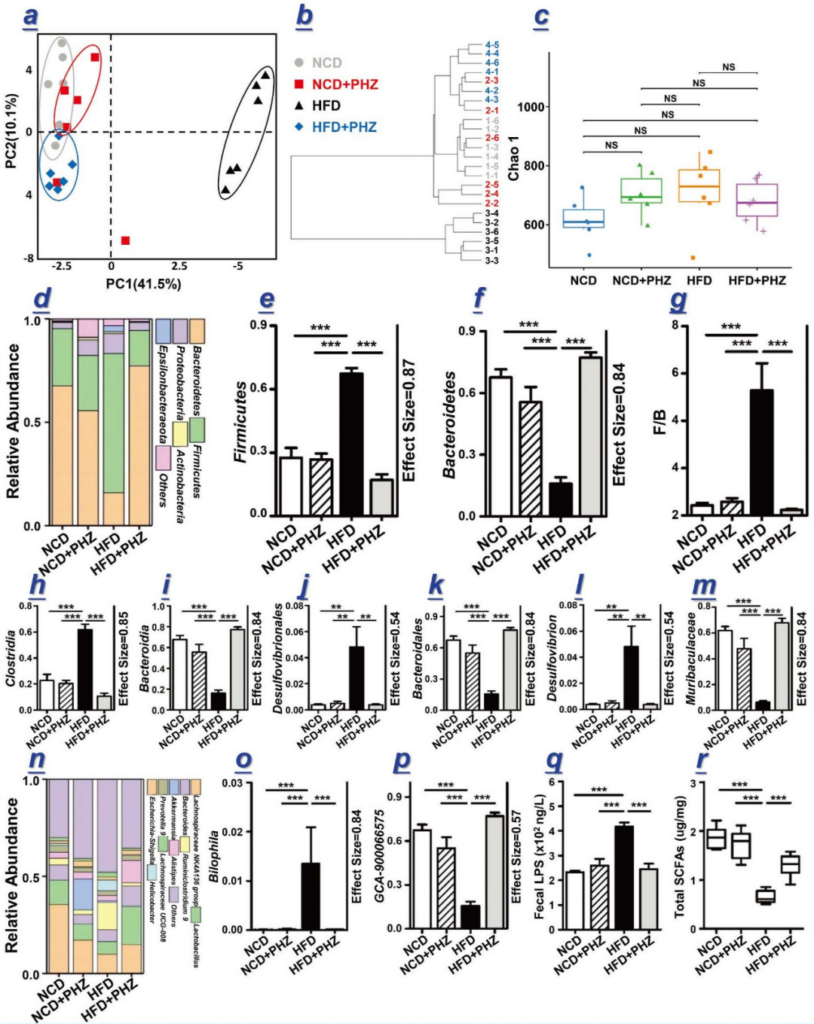
Figure 2 Phlorizin alleviates microbial and metabolic disorders induced by high-fat diet. (A) Principal Component Analysis (PCA) score chart and (b) four groups of mice [normal diet (NCD), normal diet with phlorizin (NCD + PHZ), high-fat diet (HFD) and high-fat diet with root Dermatoside (HFD + PHZ)] hierarchical clustering of fecal flora. (C) The Chao 1 index represents the alpha diversity of the intestinal flora. (D) Relative abundance of phylum level; (e, f) Relative abundance of Firmicutes and Bacteroides; (g) Ratio of relative abundances of Firmicutes and Bacteroides; (hm, op) in different The relative abundance of the differentially enriched flora identified at the taxonomic level; (n) relative abundance at the genus level; (q) fecal LPS concentration; (r) total short-chain fatty acids (SCFA). The data are expressed as mean ± standard deviation. One-way analysis of variance was used to analyze statistical differences; P> 0.05, ** P <0.01 and *** P <0.001.
To further study the specific changes in the bacterial community, we compared the relative abundance of the main phyla and genera in the four populations, especially the groups that responded to phlorizin supplementation (Figure 2d-p). At the gate level, Firmicutes and Bacteroidetes were the main gates in all groups, accounting for more than 80% of the total sequence (Figure 2d). Feeding a high-fat diet significantly increased the relative abundance of Firmicutes (P <0.001), while the addition of phlorizin significantly reversed this change (P <0.001; Figure 2e). In contrast, Bacteroidetes showed the opposite trend (P <0.001; Figure 2f).
Interestingly, in some studies, the obesity-related index Firmicutes/Bacteroidetes was higher than that of the other three groups of mice, which was higher in obese mice induced by high-fat diet (P <0.001; Figure 2g). The use of phlorizin has also shown satisfactory effects in reversing certain changes in the composition of the intestinal flora induced by high-fat diet, including Clostridia and Bacteroidia at the class level (P <0.001 in both cases; Figure 2h, i) Desulfovibrionales and Bacteroidales at the mesh level (P <0.01 and P <0.001, respectively; Figure 2j, k), Desulfovibrion and Muribaculaceae at the family level (P <0.01 and P <0.001, respectively; Figure 2l, m), and Bilophila and GCA -900066575 at the genus level (P <0.001 in both cases; Fig. 2o, p). In addition, the addition of phlorizin significantly reduced the relative abundance of the two obesity-related genera Mucispirilu and Bilophila, which were enriched by a high-fat diet (Figure 2n).
The adjustment of the above taxa may jointly help to reduce the influence of phlorizin on the changes of the overall intestinal flora. The overall regulation of intestinal flora through phlorizin supplementation can reduce the level of LPS in feces, and a high-fat diet will significantly increase the level of LPS in feces (P <0.001; Figure 2q). The detailed results of the changes in the intestinal flora can be obtained in the Supplementary Materials Figures S1-S4.
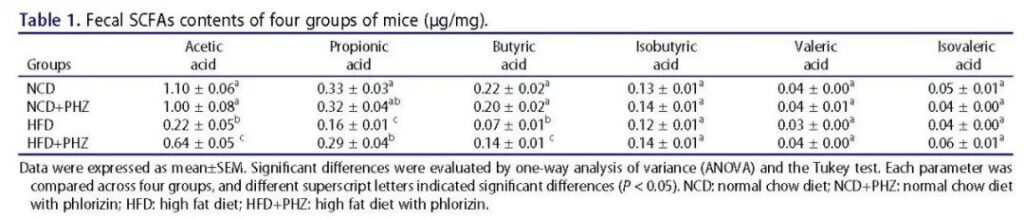
The content of short-chain fatty acids in feces was detected by gas chromatography-mass spectrometry (Figure 2r). Compared with the ordinary diet, the ordinary diet plus phlorizin and the high-fat diet plus phlorizin group, the total short-chain fatty acids in the high-fat diet group were significantly less (in all cases, P <0.001), which indicates that the high-fat diet The production of short-chain fatty acids is inhibited, and the addition of phlorizin can alleviate this situation. Specifically, compared with the high-fat diet group, the fecal acetic acid, propionic acid and butyric acid levels of the high-fat diet plus phlorizin group were significantly higher (Table 1; P <0.05). However, phloridin supplementation did not significantly change the levels of fecal isobutyric acid, isovaleric acid and valeric acid (Table 1).
Table 1 Fecal short-chain fatty acid content (μg/mg) of the four groups of mice. Data are expressed as mean±SEM. Significant differences were evaluated by one-way analysis of variance (ANOVA) and Tukey’s test. Each parameter was compared in the four groups, and different superscript letters indicate significant differences (P <0.05).
3 Phloridin improves the intestinal epithelial barrier and mucus secretion impaired by high-fat diet induction
The integrity of the intestinal epithelium is considered the first line of defense of the gastrointestinal tract. Feeding a high-fat diet may damage the intestinal epithelial barrier by increasing the intestinal LPS content. This study is consistent with previous studies that a high-fat diet can increase fecal LPS levels (Figure 2q). Histological analysis confirmed that high-fat diet caused intestinal barrier damage.
H&E and AB-PAS staining of intestinal tissues of representative mice in each group (Figure 3a, b) showed the number of goblet cells in the high-fat diet group (indicated by arrows), mucus thickness (indicated by short black lines), and intestinal wall thickness (Shown by the short blue line) and the height of the fluff (Figure 3a, b) are significantly reduced.
Phloridin supplementation can reduce the damage of epithelial integrity induced by high-fat diet, accompanied by increased blood GLP-2 levels (P <0.05, Figure 3c); GLP-2 is a peptide hormone secreted by L cells, which helps To repair and maintain the integrity of the intestinal barrier.
Quantitative histological analysis showed that compared with the high-fat diet group, the high-fat diet plus phlorizin group had villus height (P <0.01; Figure 3d), intestinal wall thickness (P <0.001; Figure 3e), and the number of goblet cells , The thickness of the mucus layer increased significantly (P <0.001; Figure 3g), indicating that phlorizin has an overall protective effect on the intestinal epithelial barrier damage induced by high-fat diet.
Table 2 The relationship between key intestinal flora and metabolites (SCFA and LPS). Statistically significant Pearson correlations are in boldface.
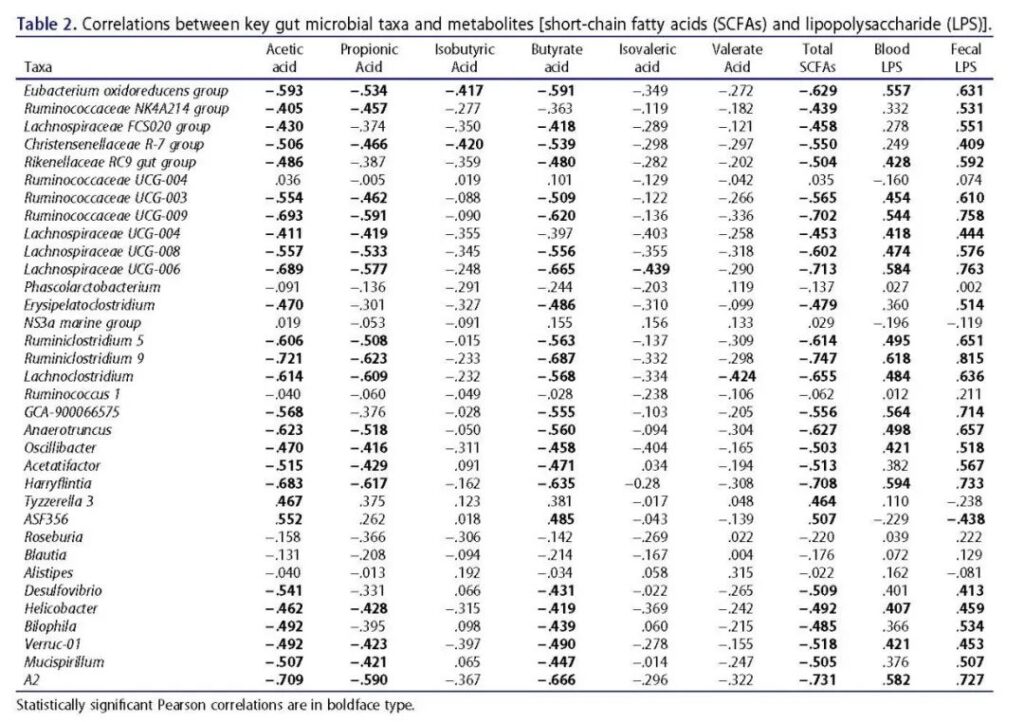
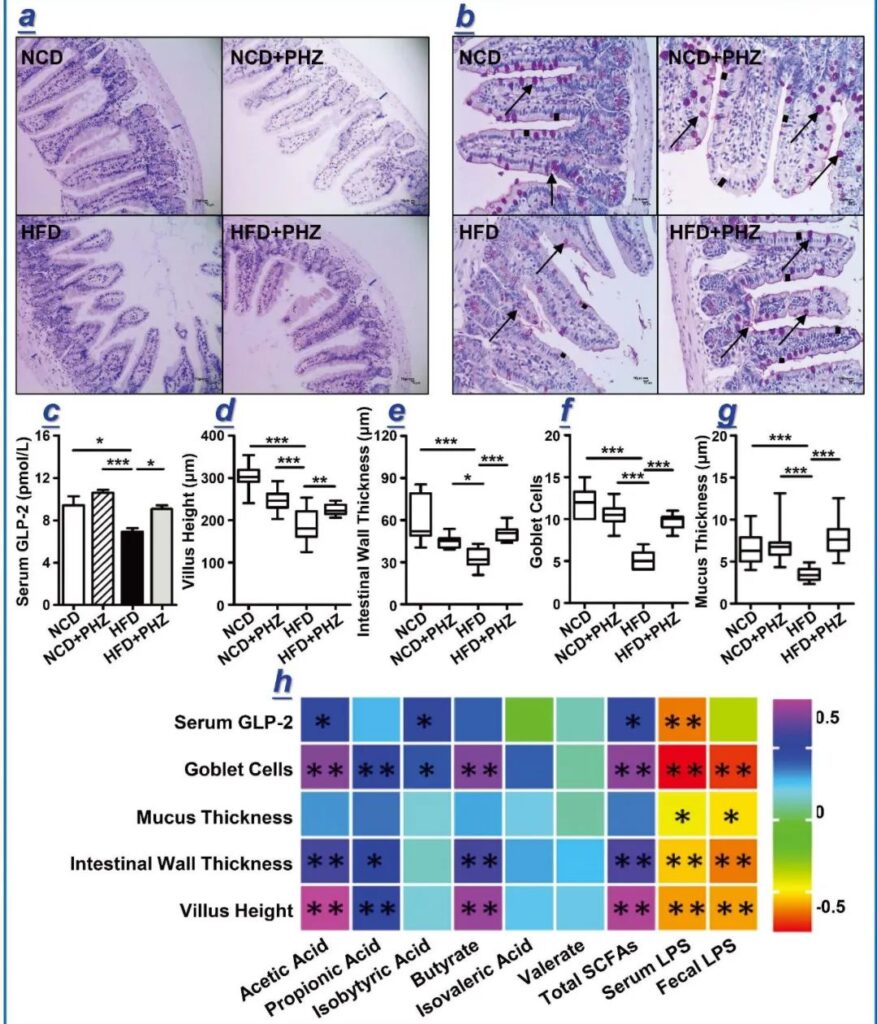
Figure 3 Phlorizin reduces the intestinal barrier damage caused by high-fat diet. (A) HE staining, (b) PAS staining. The blue mark in (a) indicates the thickness of the intestinal wall; the arrow in (b) indicates the stained goblet cells. At the 12th week, various parameters indicating the integrity of the intestinal barrier were analyzed, including (c) serum glucagon-like peptide 2 (GLP-2) level; and (d) villus height; (e) intestinal wall thickness; (F) The number of goblet cells; (g) the thickness of intestinal mucus. One-way analysis of variance was used to analyze statistical differences, * P <0.05, ** P <0.01 and *** P <0.001. The data are expressed as mean ± standard deviation. (H) Pearson correlation analysis between various fecal metabolites and intestinal epithelial integrity indicators. The color scale indicates the strength of the correlation, ranging from 0.5 (strong positive correlation) to -0.5 (strong negative correlation).
Correlation analysis was then performed to identify the association between gut microbiota/metabolites and parameters related to epithelial integrity (Table 2; Figure 3). The analysis results show that most key bacterial species are positively correlated with the content of LPS in serum and feces, and negatively correlated with the content of short-chain fatty acids, including acetic acid, propionic acid, butyric acid and total short-chain fatty acids (Table 2).
The relative abundance of these bacterial groups, for example, Eubacterium oxidoreducens group, Christensenellaceae R-7 group, Rikenellaceae RC9 gut group, Ruminococcaceae (Figure S3c), Lachnospiraceae, Desulfovibrio, Helicobacter, Bilophila (Figure S4c), and Muci4spirillum (Figure S4c) The fat diet increased significantly in mice. In the stool samples of mice in the high-fat diet plus phlorizin group, the relative abundance of these groups is usually reduced, which is most likely caused by phlorizin supplementation.
Some other details (for example, Bacillus) are not related to LPS, but are negatively related to short-chain fatty acids. In contrast, several key bacteria (including NS3a marine group, Tyzzerella 3, and ASF356) are negatively correlated with LPS and positively correlated with short-chain fatty acids; compared with mice on a high-fat diet, a high-fat diet plus phlorizin group The relative abundance of mice is significantly higher.
In addition, the results of the correlation analysis also showed that the levels of short-chain fatty acids (especially acetic acid, propionic acid, butyric acid and isobutyric acid) and intestinal barrier integrity indicators (including villus height, intestinal wall thickness, mucus thickness and goblet) There is a positive correlation between. The number of cells), and the level of GLP-2 in the blood (Figure 3h). However, indicators of intestinal barrier integrity were negatively correlated with LPS levels (Figure 3h).
4 The transplantation of the intestinal flora of mice in the high-fat diet plus phlorizin group reduced the damage of intestinal epithelial integrity and obesity induced by the high-fat diet
In order to confirm that the reduction of the adverse reactions induced by the high-fat diet was caused by the intestinal flora rather than the residual phloridin (and its metabolites such as phloretin) in the feces of the donor mice, HPLC was used.
Targeted chemical analysis of the spectra of phloridin-related compounds in feces collected quantitatively before fecal bacteria transplantation. The two peaks corresponding to the phloridin and phloridin standards appeared on the HPLC chromatogram of the standards, but they were not detected in the stool samples of the four groups of donor mice, indicating that there was no phloridin or phloridin in these samples. Phloretin (Figure 4a).
The feces of the four groups of donor mice were transplanted into a new batch of mice adapted to the environment every day, and then maintained on a high-fat diet for eight weeks, and their weight changes were monitored (Figure 4b). In the eighth week, many parameters related to obesity and intestinal epithelial barrier integrity were detected, including the weight of epididymal fat and liver (Figure 4c), H&E staining of epididymal fat (Figure 4d), and adipocyte size of epididymal fat (Figure 4e) ), liver morphology (Figure 4f), fasting blood glucose and insulin (Figure 4g, h), serum GLP-2, diamine oxidase (DAO) and D-lactic acid (Figure 4i-k), H&E and AB of intestinal tissue section -PAS staining (Figure 4l, m).
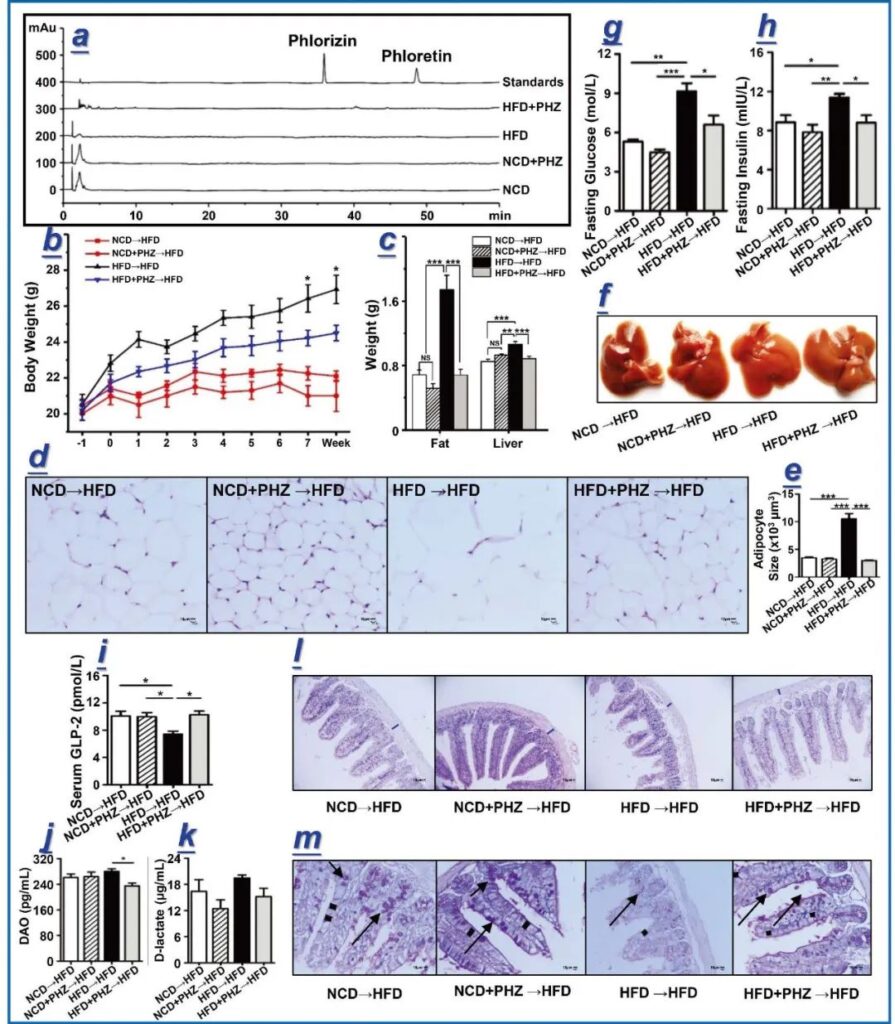
Figure 4 Phlorizin faecalis transplantation alleviates obesity and intestinal barrier damage caused by high-fat diet. Four groups of donor mice fed with normal diet (NCD), normal diet supplemented with phlorizin (NCD + PHZ), high-fat diet (HFD) and high-fat diet supplemented with phlorizin (HFD + PHZ). And phloretin (PHT) chromatogram. The four groups of recipient mice (fed with high-fat diet) undergoing fecal microbiota transplantation were named “NCD→HFD”, “NCD + PHZ→HFD”, “HFD→HFD” and “HFD + PHZ→HFD” . (B) Weight change at 8 weeks.
In the eighth week, many parameters related to obesity and intestinal epithelial barrier integrity were recorded, including (c) the weight of epididymal fat and liver; (d) hematoxylin and eosin (H&E) staining of epididymal fat; (e) The size of fat cells in epididymal fat; (f) liver morphology; (g, h) fasting blood glucose and insulin levels; (ik) glucagon-like peptide 2 (GLP-2), diamine oxidase (DAO) and D- Serum levels of lactate; (l, m) H&E and PAS staining of intestinal tissue sections. The blue mark in (l) indicates the thickness of the intestinal wall; the arrow in (m) indicates the stained goblet cells. The data are expressed as mean ± standard deviation. One-way analysis of variance was used to analyze statistical differences; P> 0.05, * P <0.05, ** P <0.01 and *** P <0.001.
Stool transplantation from mice in the high-fat diet plus phlorizin group can significantly reduce the weight gain induced by the high-fat diet and the increase in blood glucose of the recipient mice, accompanied by improvement in insulin resistance and a decrease in serum LPS, which indicates that phlorizin Regulated flora can attenuate the metabolic syndrome induced by high-fat diet and the intestinal flora is the goal of improving the effect of phlorizin (Figure 4 and S6).
Our conclusions also showed that the serum GLP-2 level of fecal transplant mice obtained from the high-fat diet plus phlorizin group was significantly higher than the serum GLP-2 level of the fecal mice transplanted from the high-fat diet group (P <0.05 ; Figure 4i), which indicates that phlorizin can stimulate the secretion of GLP-2. Moreover, after transplanting the phlorizin-regulated microbiota into mice fed a high-fat diet, the impairment of intestinal epithelial integrity induced by the high-fat diet was also alleviated, which was confirmed by the microscopic morphology of the intestinal tissue ( Figure 4l, m), and in the intestinal wall thickness (Figure S6g), villi height (P <0.01) (Figure S6h), the thickness of the mucus layer (P <0.001; Figure S6i) and the number of goblet cells (P <.01) ; Figure 4m and S6j) have also been confirmed.
The permeability and function of the intestinal barrier were evaluated by the serum levels of Diamine Oxidase and D-lactic acid. Diamine Oxidase enzyme is usually located in the upper villi of the mucosa of the small intestine. D-lactic acid is a metabolite of intestinal bacteria produced in the intestine, and it cannot be metabolized by mammals due to the lack of the corresponding fast enzyme system. Therefore, serum Diamine Oxidase and D-lactic acid levels can reflect the permeability and integrity of the intestinal epithelium. As shown in Figure 4j, k, the serum levels of Diamine Oxidase were 261.63 ± 10.60 pg/mL and 280.25 ± 7.96 pg/mL in the stool transplanted mice obtained from the normal diet group and the high-fat diet group.
This indicates that high-fat diet mice transplanted with feces can increase the serum levels of Diamine Oxidase in recipient mice. The increase in serum D-lactic acid was similar in mice receiving the feces of ordinary diet mice and mice in the high-fat diet group, which were 16.37 ± 2.68 μg/mL and 19.44 ± 0.68 μg/mL, respectively. These results indicate that the intestinal barrier of the mice receiving the high-fat diet mouse feces transplantation is higher than that of the mice receiving the normal diet. However, compared with mice extracted from the feces of mice on a high-fat diet plus phloridin, the recipients of fecal transplantation from mice on a high-fat diet plus phloridin had lower serum Diamine Oxidase and lactic acid levels (respectively 235.08 ± 8.71 pg/mL and 15.18 ± 1.89 μg/mL). The transplantation of phloridin-regulated flora can alleviate the intestinal barrier damage induced by high-fat diet.
Discussion:
The results of this paper show that phlorizin supplementation can significantly inhibit the weight gain and fat deposition in obese mice induced by high-fat diet, and it is accompanied by a reduction in serum LPS levels and an improvement in insulin resistance. After oral administration, phloridin is mostly absorbed through the gastrointestinal tract. Therefore, this study tested the following hypothesis: the intestinal epithelial barrier and intestinal flora are potential new targets for the pharmacological effects of phlorizin. By using a high-fat diet to induce obesity in a mouse model and fecal bacteria transplantation, this study shows that the “intestinal flora-barrier axis” is indeed the target of phloridin’s anti-obesity effect.
This study shows that the intestinal flora is a potential target for phlorizin to improve the metabolic syndrome, partly evidenced by the decrease in intestinal LPS and the increase in short-chain fatty acid levels. LPS and short-chain fatty acids are two metabolites derived from the intestinal flora and are widely regarded as classic indicators of the state of the intestinal system and the function of the intestinal flora. The results of this study show that phlorizin can significantly reduce the level of certain LPS production and obesity-related bacteria in obese mice induced by high-fat diet, such as Desulfovibrio, Ruminiclostridium, Anaerotrucus and Oscillibacter, resulting in a decrease in the overall level of fecal LPS.
Correlation analysis showed that the flora of obese mice induced by high-fat diet was positively correlated with LPS, but negatively correlated with short-chain fatty acids. The addition of phlorizin reduced the increase of LPS and the decrease of short-chain fatty acids. High-fat diet induces an increase in bacteria such as Eubacterium oxidoreducens group, Christensenellaceae R-7 group, Rikenellaceae RC9 gut group, Ruminococcaceae, Lachnospiraceae, Desulfovibrio, Helicobacter, Bilophila and Mucispirillum at the level of obese mice. These bacteria are added to the high-fat diet with root bark Significantly decreased in the glycoside group. On the other hand, co-administration of phlorizin can significantly promote the growth of bacterial groups that are positively related to short-chain fatty acids but negatively related to LPS (for example, NS3a marine group, Tyzzerella 3 and ASF356).
LPS is considered to be one of the main causes of chronic inflammation and even metabolic syndrome. It can break and penetrate the intestinal barrier, especially after binding to chylomicrons. The damage of intestinal barrier function is related to various intestinal and systemic diseases. Therefore, the authors speculate that phlorizin can cause a large reduction in LPS-producing bacteria and help reduce LPS and subsequent damage to the intestinal physical intestinal barrier, thereby limiting the leakage of LPS to the blood.
Another important class of microbial metabolites involved in the anti-obesity effect of phlorizin is short-chain fatty acids. Short-chain fatty acids play a vital role in regulating the physiological functions of the host and intestinal homeostasis, especially enhancing the integrity of the intestinal barrier. This study found that feeding phlorizin to obese mice induced by a high-fat diet can significantly alleviate the reduction of short-chain fatty acids in the feces of these mice, especially acetic acid, propionic acid and butyric acid. In fact, it is reported that short-chain fatty acids directly participate in hormone secretion and promote the differentiation and activity of L cells, which are distributed throughout the epithelial layer of the intestinal wall. Some hormones regulated by short-chain fatty acids include GLP-1 and GLP-2. GLP-1 enhances the secretion of glucose-dependent insulin, and GLP-2 is known to be involved in repairing and maintaining the integrity of the intestinal barrier. Therefore, another possible mechanism of the anti-obesity effect of phlorizin may be to further regulate hormone secretion by stimulating bacteria that produce short-chain fatty acids and subsequently increasing the content of short-chain fatty acids in the colon, thereby leading to the restoration of the intestinal barrier and the reversal of insulin resistance.
In order to further test the hypothesis that the intestinal barrier and intestinal flora are the targets of the anti-obesity effect of phlorizin, the feces of mice fed with ordinary or high-fat diet were transplanted and administered with or without phlorizin. Feces were tested for fecal bacteria transplantation. Before fecal bacteria transplantation, HPLC analysis was performed to confirm the absence of phloridin and related compounds in the stool samples to be transplanted to recipient mice. In fact, the ingested phloridin will enter the gastrointestinal tract and may act through a two-step mechanism: 1. Most of the unabsorbed phloridin reaches the gastrointestinal tract, and some of it is metabolized into phloretin. 2. These compounds locally modulate the intestinal flora in a similar way to prebiotics. As a result, the production of LPS is inhibited, and the damage of LPS to the intestinal epithelial barrier is reduced. At the same time, the level of short-chain fatty acids increases, which in turn stimulates the secretion of GLP-2. GLP-2 then helps repair the damaged physical barrier of the gastrointestinal tract and effectively prevents LPS from penetrating into the systemic circulation and endotoxemia. Although the bioavailability of phloridin is low and it competitively inhibits the absorption of SGLTs into the bloodstream, our findings support the combined effects of certain effector molecules (such as short-chain fatty acids and GLP-2) to help improve A high-fat diet induces an improvement in metabolic syndrome such as insulin resistance (Figure 5).
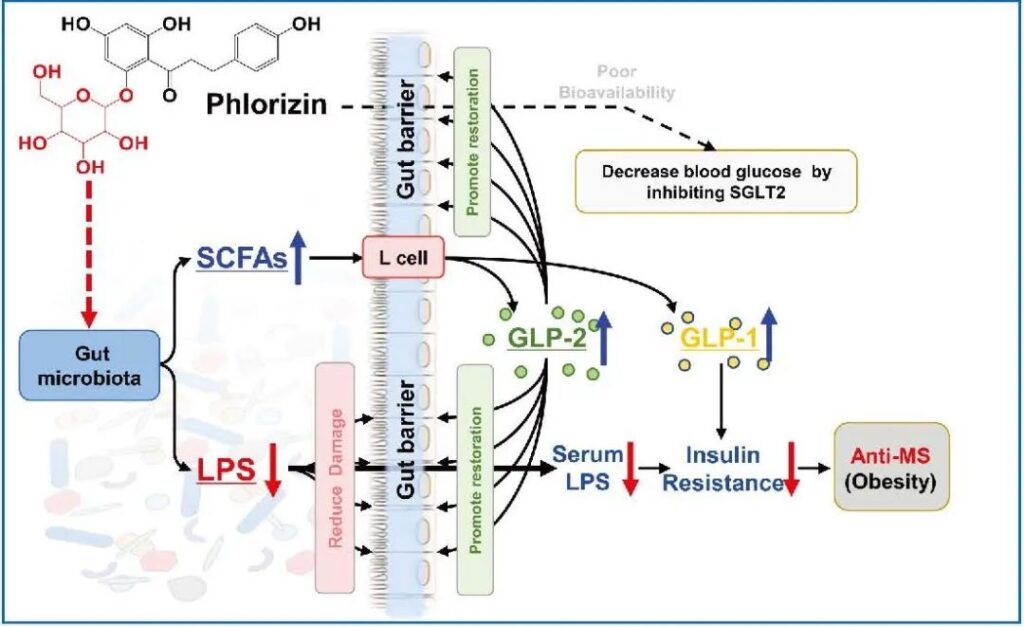
Figure 5 The possible mechanism of phlorizin to improve metabolic syndrome. The schematic diagram shows the possible mechanism of phlorizin to improve metabolism. In addition to SGLT2, the “intestinal flora-barrier axis” including the intestinal flora and the intestinal barrier is the target for phloridin to play a beneficial role.
Sum up:
There is increasing evidence that polyphenols of plant origin (Including flavonoids, phenolic acids, hydroxyphenols and catechins) can effectively prevent and treat metabolic syndrome by improving the antioxidant and anti-inflammatory status of the intestine, thereby enhancing the epithelial barrier function and regulating the intestinal flora. The transplantation experiment of fecal bacteria in this study showed that the intestinal epithelial barrier and intestinal flora are alternative targets for the therapeutic effect of phlorizin.
However, it is worth noting that in the application of fecal bacteria transplantation, more attention should be paid to the identification of metabolites derived from microorganisms and polyphenols in donor feces to ensure biological safety. Previous metabolomics analysis has found that functional effectors of beneficial functions are usually not polyphenols, but their metabolites. For example, most natural compounds in the glycoside form will need to be activated to a more biologically active form of aglycone through the first hydrolysis step.
Similarly, after entering the gastrointestinal tract, phloretin is usually hydrolyzed by acid and/or enzymes to phloretin. Therefore, it is currently unclear whether the anti-obesity and intestinal microbiota regulation effects of phloridin are caused by the original form of phloridin and/or phloretin. In particular, the preliminary study of this study found that the in vitro antibacterial effect of phloretin is stronger than that of phlorizin (data not shown).
In addition, the unabsorbed phlorizin/phloridin may enter the digestive tract and interact with the intestinal flora. On the one hand, phlorizin and phloretin can regulate the composition of the intestinal flora. On the other hand, phlorizin and phloretin can be used as substrates metabolized by intestinal microbes, and then release other metabolites to stimulate downstream metabolism. . Finally, to confirm the role of the intestinal flora and its role as the target of phlorizin in the anti-obesity effect, it is important to ensure that there are no phlorizin and phloretin residues in the feces to be transplanted.
The dose of phloridin is sometimes excreted and detected in stool samples. It is also important to ensure that there are no other glycosides and/or phenols with low bioavailability in the feces of the recipient mice, such as phlorizin; otherwise, it may be difficult to verify whether the intestinal flora can serve as root bark Alternative targets for glycosides, or it is difficult to understand the role of phlorizin in anti-obesity.
Phloridin (PHZ) improves obesity-related endotoxemia and insulin resistance
Phloridin (PHZ) improves obesity-related endotoxemia and insulin resistance
Phloridin (PHZ) improves obesity-related endotoxemia and insulin resistance
(source:internet, reference only)
Disclaimer of medicaltrend.org