CAR-T therapy: The total remission rate of tumors is nearly 100% !
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
CAR-T therapy: The total remission rate of tumors is nearly 100% !
CAR-T therapy: The total remission rate of tumors is nearly 100% ! Long-term follow-up data released! The total remission rate is nearly 100%! Universal .
In the long history of humans fighting cancer, chimeric antigen receptor T cell (CAR-T) cell therapy is absolutely a breakthrough therapy, occupying half of the tumor immunotherapy, especially in the area of hematological tumors. In 2017, the only two CAR-T cell therapy commercial products in the world were approved: Novartis’ Kymriah (lymphoma) and Gilead’s Yescarta (leukemia), which can be said to have completely changed the pattern of cancer treatment.
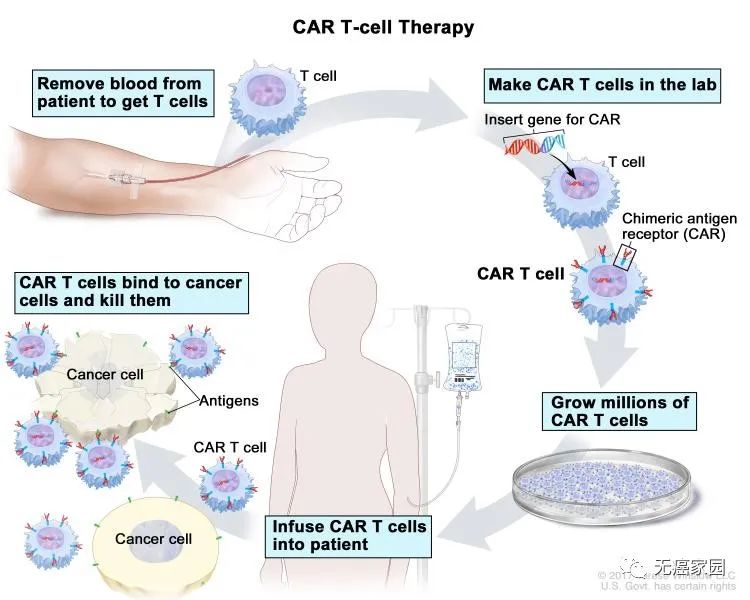
At the American Association for Cancer Research (AACR) meeting in 2021, CAR-T therapy is in full swing and has achieved breakthrough results in the treatment of hematological tumors. Then let the editor introduce the latest research announced at the meeting. Results.
The remission rate is nearly 100%! The data of universal CAR-T therapy for T-ALL is amazing!
On April 10 this year, China Genxi Biotechnology Group announced its universal TruUCAR GC027 for the treatment of relapsed or refractory (R/R) acute T-lymphocytic leukemia (T-ALL) adult patients at the 2021 AACR meeting. The latest long-term follow-up data.
This clinical trial is the first human trial initiated by researchers to evaluate the safety and effectiveness of the universal TruUCAR GC027 in the treatment of R/R T-ALL patients. At the AACR2020 annual meeting, the trial investigator announced the results of the study for the first time in the form of an oral report.
The latest data as of February 4, 2021, shows the long-term follow-up status of subjects who were initially enrolled and subsequently enrolled. All patients received a single infusion of TruUCAR GC027, and the infusion measurement was one of the three measurement groups of high, medium and low: 0.6*10^7 cells/kg, 1.0*10^7 cells/kg, or 1.5*10 ^7 cells/kg.
All 6 (100%) subjects
Reach
totally relaxed
, Including complete or incomplete recovery of the number of blood cells (CR/CRi); among them,
5 (83%)
The patient has reached
Complete remission with negative minimal residual disease
(MRD-CR). in
Received treatment for 6 months
After that
3 out of 5 patients (60%)
Still maintain the MRD-CR status.
The results of the 18.5-month follow-up of the first 5 subjects showed:
(1) 1 patient still maintained MRD-CR status at 16.8 months;
(2) One patient maintained the MRD-CR status for 9 months;
(3) A patient with primary refractory disease (no response to VDP chemotherapy regimen) maintained the MRD-CR status for 7 months.
(4) In addition, another patient initially showed high tumor burden and extensive extramedullary lesions. After receiving TruUCARGC027 therapy, PET-CT scan confirmed that all extramedullary lesions were cleared, and the patient was on the 28th day. Reach the MRD-CR status.
All 6 patients tolerated a single infusion of TruUCARGC027, and no neurotoxic events (ICANS) or acute graft-versus-host disease (aGvHD) were observed.
All patients had different degrees of cytokine release syndrome (CRS), but they were effectively controlled after standard treatment including tocilizumab. The overall safety is consistent with previously observed results.
According to Dr. Wang Lihong from Cancer Free Home Network, T-ALL is an acute lymphoblastic leukemia (ALL), which accounts for 12% to 15% of childhood ALL, and up to 20% to 25% of adult ALL. %, it is a refractory disease with very limited treatment methods, and most T-ALL patients are prone to relapse within 2 years after multiple drugs combined with chemotherapy.
GC027 uses Genxi’s unique TruUCAR patented technology and uses healthy donor T cells that do not require HLA matching. It is a ready-to-use CAR-T cell therapy under development that can target tumor cells expressing CD7 antigen. For the treatment of T cell malignant tumors.
In addition, TruUCAR technology allows allogeneic CAR-T cells to proliferate and persist in patients (recipients) with HLA mismatches, while controlling the risk of graft-versus-host disease (GvHD).
More importantly, TruUCAR is a ready-to-use type, not only can patients be treated in time, but also its low manufacturing cost makes repeated administration possible!
Blood tumor! Solid tumor! The new CAR-T therapy can’t let go
In addition to the aforementioned breakthrough clinical research on CAR-T therapy in hematological tumors, the country is currently actively carrying out CAR-T clinical research related to hematological tumors and solid tumors.
The emergence of CAR-T cell immunotherapy has given the majority of cancer patients hope. Cellular immunotherapy represented by CAR-T therapy will be the hottest field in the global biopharmaceutical industry in the next 5 to 10 years.
The editor believes that in the near future, more and more clinical trial data will continue to emerge. CAR-T therapy will not only shine in the field of hematological tumors, but also increasingly demonstrate its hidden strength in solid tumors!
(source:internet, reference only)
Disclaimer of medicaltrend.org



