New TILs therapy treats advanced melanoma with 80.3% DCR
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New TILs therapy treats advanced melanoma with 80.3% DCR
New TILs therapy treats advanced melanoma with 80.3% DCR, and the patients with advanced stages are saved!
Many patients do not know how precious the tumor tissue removed after surgery is!
When hearing about advanced cancers and metastatic tumors, many people feel hopeless to survive. In fact, more and more advanced cancer patients can also achieve long-term survival.
Especially many patients do not know how precious the tumor tissue removed after surgery is!
With the continuous development of tumor immunology research, tumor immunotherapy has become the fourth largest tumor treatment after surgery, radiotherapy, and chemotherapy. In fact, a group of immune cells with the strongest cancer-killing ability-tumor-infiltrating lymphocytes is hidden in the tumor tissues surgically removed from patients.
Scientists have found that in the early stages of cancer, the immune system tries to mobilize some special lymphocytes to attack tumors. These lymphocytes have the ability to recognize and attack tumors and can penetrate deep into tumor tissues. Scientists named them tumor infiltrating lymphocytes (TILs).
TILs therapy, to put it simply, is to separate and purify the lymphocytes in the surgically resected tumor tissue, select the lymphocytes that can specifically anti-cancer, and reinfuse after amplification and activation. This type of therapy has a history of more than 30 years and was first used for malignant melanoma. Then the editor will lead you cancer friends to see the latest research progress.
The DCR is as high as 80.3%, and the follow-up time is extended to 28.1 months. TILs therapy is expected to be available this year!
At the 2021 AACR meeting that just came to an end, it was reported that autologous tumor infiltrating lymphocytes can produce a long-lasting response in pretreated advanced melanoma, that is, the innovative therapy based on TILs cells, LN-144 (Lifileucel), has shown positive results After more than 28 months of follow-up for patients with advanced melanoma, the median response duration has not yet been reached. Once this result was issued, it caused a sensation again!

C-144-01 is a phase 2 clinical trial that recruits 66 patients who have been diagnosed with stage IIIc or stage IV metastatic melanoma. The characteristics of the patients are those who have received at least 3 to 4 systemic treatments after failure, and have also received PD-1 inhibitor treatment. Among them, 80% of patients received CTLA-4 inhibitor therapy, and 23% of patients received BRAF/MEK inhibitor combination therapy.
The treated patients also had a high tumor burden at baseline, with an average target lesion diameter of 10.6 cm, 77% of target and non-target lesions had more than 3, and 42% had liver and/or brain lesions.
Bright data:
1. The disease control rate (DCR) is as high as 80.3%;
2. The objective response rate (ORR) reached 36.4%; (including 4.5% complete remission and 31.8% partial remission), patients with stable disease accounted for 43.9%.
3. The median follow-up time was 28.1 months (the AACR announced in 2020 was 8.8 months), and the median duration of response was not reached.
4. It needs to be pointed out that patients with PD-L1 negative also respond, which means that patients who are ineffective against immune checkpoint inhibitors can still benefit from TILs therapy.
Expert Reviews:
These patients basically have exhausted their treatment options clinically, and 42% of patients who have entered the very advanced stage even have liver or brain metastases.
The effect of this TILs therapy is really surprising, especially for patients who have progressed after PD-1 treatment and have no better treatment options.
【Typical cases】
A patient with advanced melanoma had extensive tumor metastasis before treatment. One month after receiving TILs therapy, the focus was significantly reduced, and complete remission was achieved within 6 months of treatment. After two years of treatment, it was still in complete remission, and tumor responsiveness persisted in the body. CD8+ T cells.
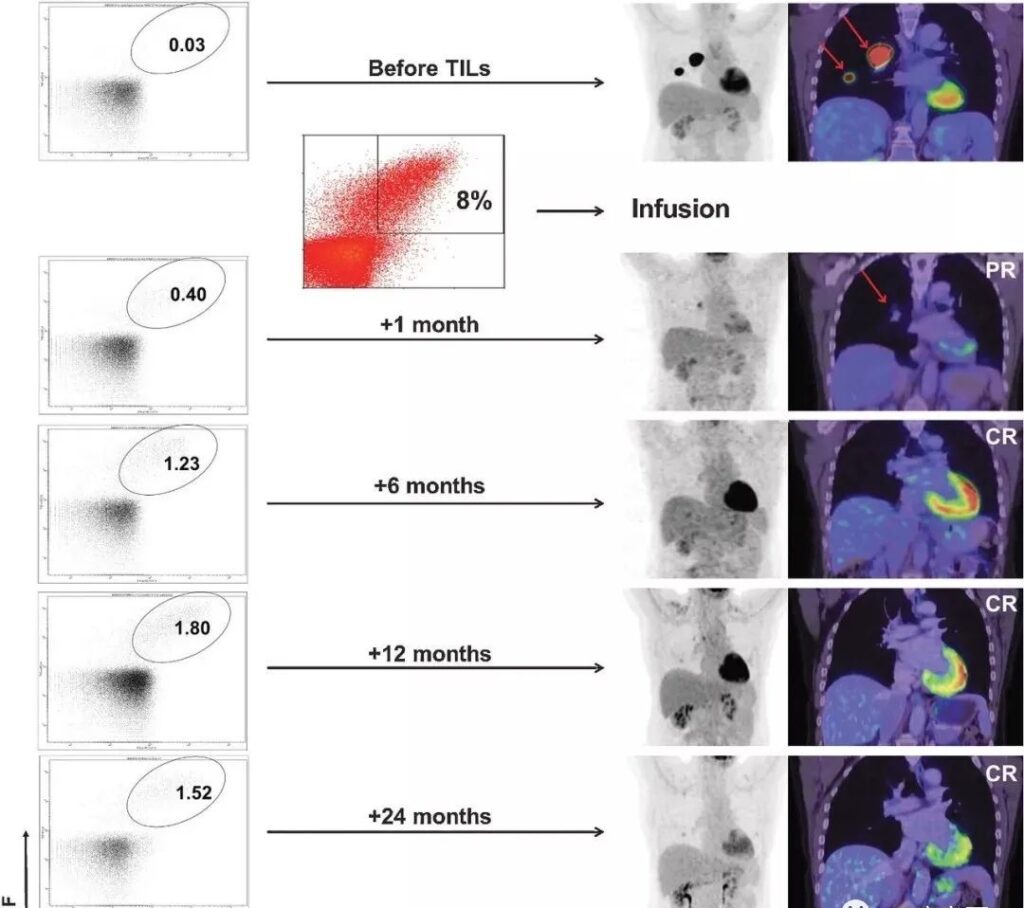
Patient story
The above research follow-up time is cold, but in order to be more convincing, the editor specifically consults relevant foreign patient cases and tells the story of the treatment of melanoma patients with TILs therapy.
If you want to survive, participating in a clinical trial is the only option. After receiving TILs treatment, it will be cancer-free for 7 years
As early as 4 years ago, Jamie Goldfarb (Jamie Goldfarb) was diagnosed with stage II melanoma. In the second year, the tumor progressed to stage III, but the operation was successful and the subsequent PET examination was normal. The story should have ended in this way, but this is not the case.
Unfortunately, her melanoma recurred. She was diagnosed with stage IV melanoma in January 2011 and spread to her liver and pancreas. At that time, her son was only 11 weeks old.
Jamie understands that if she wants to survive, participating in a clinical trial seems to be the only choice, and which clinical trial to participate in is the problem she faces.
After consulting key opinion leaders in the field of melanoma (such as Dr. Stephen Rosenberg, director of the Department of Surgery at the National Cancer Institute and members of the MRA Scientific Advisory Group), the feedback was unanimous that if she is eligible to participate in the NCI TILs trial , This is her best choice.
After receiving TILs for 4 months, the situation began to change, and she clearly felt the tumor shrink. Scan by scan and scan by month continue to show significant improvement. By 2014, only two years after TILs treatment, she was declared as having no visible lesions.

“I can live to this day thanks to participating in the TILs clinical trial. The five-year survival rate for stage IV melanoma is only 14%, and the prognosis is very poor. TILs treatment saved my life.” Jamie said. Seven years have passed, and she still survives cancer-free.
No recurrence in 9 years, TILs therapy is very effective in treating melanoma!
In 2011, the Netherlands Cancer Institute (NKI) launched a phase I/II feasibility study for TIL in the treatment of metastatic melanoma to evaluate the feasibility and clinical effectiveness. The relevant results were recently published in the Journal for immunotherapy of cancer. .
A total of 10 patients received treatment, and 5 showed obvious clinical response, including 2 patients in complete remission, and continued treatment for more than 7 years. One patient had stable disease for more than 2 months, and the remaining 4 showed progressive disease.
Among them, patient 3 with the best treatment effect was a 46-year-old male with BRAF V600E mutation-positive melanoma, accompanied by lymph node metastasis, liver metastasis, and left adrenal gland enlargement.
During the treatment of verofenil, the patient’s liver metastases completely disappeared, but lymph node metastasis progressed. He was subsequently treated with ipilimumab, but showed progressive disease after 4 courses. At the time of study entry, the patient showed large metastases in his right upper leg, as well as lymph node metastasis, adrenal enlargement, but no signs of liver, lung, or brain metastasis. After that, the patient received 19.6×10^10 TIL, followed by 4 large doses of IL-2, and complete remission was achieved 12 weeks after TILs infusion.
After TIL treatment, the patient remained disease-free for more than 9 years. The following figure shows the best response of 10 patients. Among them, the picture of patient 3’s leg tumor gives the strongest proof of the efficacy of neoantigen TILs.
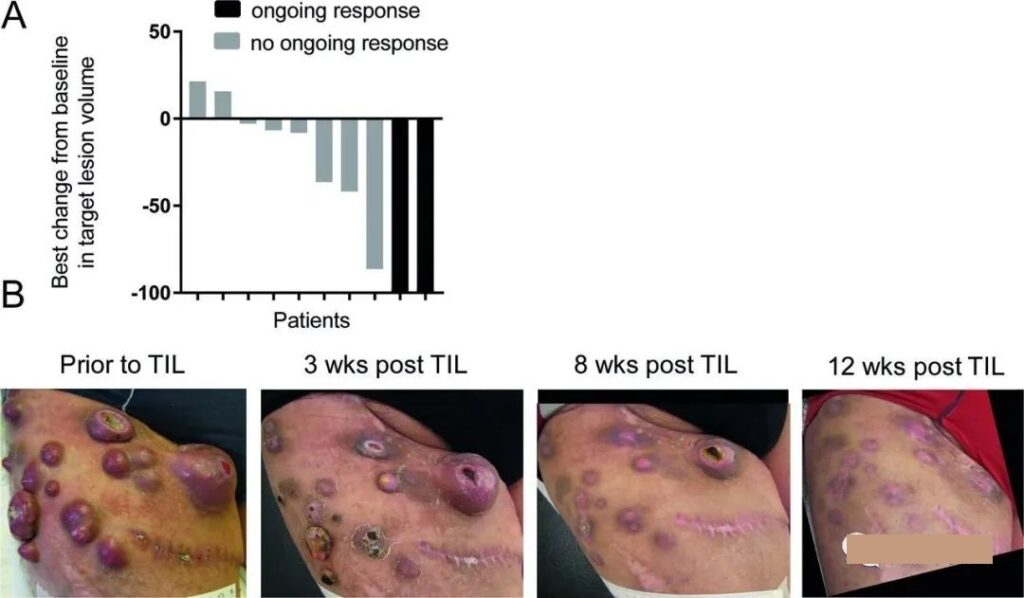
TILs therapy is the first to declare war on solid tumors and is expected to be available soon!
In addition to the good therapeutic effects of TILs therapy in the field of melanoma, in recent years, good data has been given in various solid tumors such as cervical cancer and lung cancer.
1 Lung cancer: open long survival period, TIL therapy brings hope to PD-1 resistant patients
At the 2020 AACR meeting, a phase I clinical result of advanced metastatic non-small cell lung cancer treated with TILs:
A total of 16 patients in this trial were treated with TILS, of which 12 patients could be evaluated.
At an average follow-up of 1.4 years, 3 patients were in remission, and 2 of them were in complete remission for more than one year. The remission of the other patient is to be confirmed.
After most patients received TILs treatment, the tumor lesions have shrunk.
At the first CT scan after treatment, the tumor lesion diameter was reduced by an average of 38%!
2 Cervical cancer: 89% disease control rate! LN-145 therapy won the FDA breakthrough therapy title!
In June 2019, the FDA approved the tumor-infiltrating lymphocyte (TIL) treatment method LN-145 as a breakthrough treatment designation. This is the first time that a cellular immunotherapy for solid tumors has won this award. I believe that it is only a matter of time before it goes to market.
Once approved by the FDA, this will be the first cellular immunotherapy for solid tumors and will bring huge survival benefits to cancer patients.
The latest data show that the objective response rate (ORR) is 44%, the complete response rate (CR) is 11%, and the disease control rate (DCR) is as high as 85%.
3 Cholangiocarcinoma: The first super survivor to receive TIL therapy has successfully crossed 11 years
2009 was unforgettable for Melinda Bachini. On the day of her son’s 14th birthday, she was diagnosed with a rare bile duct cancer. Dr. Steven Rosenberg of the National Cancer Institute of the United States unfortunately told her that when the survival period was only a few months, she felt as if she had fallen into an ice cave.
However, life will always experience great ups and downs. The amazing thing is that she is now not only promoted from a mother of 6 children to a grandmother, but also a survivor of advanced cholangiocarcinoma for more than ten years!
This blessing is all due to her participation in the latest immunotherapy clinical trial developed by Dr. Steven Rosenberg’s team in the United States.
In the trial, the research team used highly personalized tumor infiltrating lymphocyte TILS therapy to cure Melinda Bachini’s tumor. A major breakthrough was reported in the “Science” magazine, and her anti-cancer experience has also brought confidence to countless patients.
Expert comments:
In addition, TILs therapy also shows great potential in colorectal cancer, breast cancer, head and neck, sarcoma and other malignant tumors. Please check the relevant links at the end of the article.
Various studies that have been developed indicate that TIL therapy will need to be continuously improved and developed, and will eventually become a new weapon for human anti-cancer.
Currently, TILs therapy is expected to submit a marketing application in 2021. Once approved by the FDA, this will be the first cellular immunotherapy for solid tumors and will bring huge survival benefits to cancer patients.
(source:internet, reference only)
Disclaimer of medicaltrend.org



