China SINOPHARM COVID-19 Vaccine is not safe for Seniors or Women ?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
China SINOPHARM COVID-19 Vaccine is not safe for Seniors or Women ?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
China SINOPHARM COVID-19 Vaccine is not safe for Seniors or Women ?
Data of China SINOPHARM COVID-19 Vaccine Finally Released! WHO is evaluating if it meets the standard for emergency use.
SINOPHARM is one of the most concerned vaccines because of its widespread vaccination. In general, the current clinical trial data cannot explain that the SINOPHARM vaccine is safe and effective in the elderly and patients with underlying diseases. What’s more surprising is that among the volunteers enrolled in SINOPHARM, women accounted for only about 15% (only 2167 women out of 13,765 vaccinators).
Vaccines of China SINOPHARM and Kexing (SINOVAC) should be the most vaccinated and the most authorized countries. The effectiveness and safety of these two vaccines are of the greatest concern to everyone.
On April 29, WHO spent a special day to evaluate whether the SINOPHARM and KEXING vaccines meet the WHO emergency authorization standards for COVID-19 vaccines.
Now that the effectiveness and safety data presented by SINOPHARM at the conference have also been made public on the WHO website, we might as well take a look at this most mysterious COVID-19 vaccine.
WHO Evidence Assessment:
Please visit WHO link to downloand: Evidence Assessment: Sinopharm/BBIBP COVID-19 vaccine
Don’t confuse test results for different purposes
It is not easy to make a vaccine, and it is also a big project to complete the verification of the vaccine’s effectiveness and safety. The results of light clinical trials will involve different “categories”.
When everyone is tracking the development of the COVID-19 vaccine, some information content unfortunately does not explain the results of different categories clearly. The most common one is the misunderstanding of the antibody conversion rate as effectiveness.
Such misunderstandings can easily confuse people. The SINOPHARM evaluation report of WHO has done a good job on this point. The first data summary is to show the different purposes of the experiment and the corresponding data volume in categories.
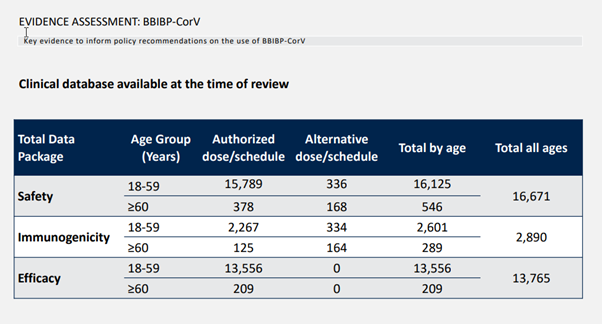
Looking at the above figure, three categories of clinical trial data, safety, immunogenicity, and effectiveness, are clearly classified, and the corresponding numbers are clearly written in terms of young people under 60 and old people over 60. Note that these are all vaccinated people, and those who received a placebo are not counted.
Let’s talk about immunogenicity first, which is the result of antibody conversion rate and neutralizing antibody titer that we often see.
This is to examine whether the vaccine causes an immune response in the human body and how strong the immune response is.
It should be noted that for a vaccine, good immunogenicity, such as 100% antibody conversion rate and high induced antibody titer, are necessary but not sufficient conditions for the vaccine to be effective. In other words, a vaccine that can’t even activate the immune response will definitely be useless. But just based on the immune response, we cannot directly infer that the vaccine must be effective.
It should also be noted that immunogenicity is generally measured in the laboratory with blood samples of healthy volunteers. Therefore, the number of people involved is relatively small, and there are often no people with underlying diseases. Like SINOPHARM, there are only 2890 people with immunogenicity data, and 289 people over 60 years old.
If immunogenicity will be the least number of people, then the most number of people is generally safety. Even from the same phase III clinical trial, the number of people involved in safety data will be higher than that of effectiveness. Why? Because the effectiveness of the vaccine is mainly calculated from two weeks after the second injection, the safety begins after the group is given the injection. Volunteers are enrolled in the group first, and those who come late may not be placed in the validity calculation group when analyzing the data. Naturally, the number of safety data is larger than the validity.
But this is also good. After all, the most basic thing about a vaccine is to be safe, and the more data in this area, the better. How many people does SINOPHARM have safety data? 16,671 people, the total number is not bad, after all, like Moderna’s Phase III clinical trial of 30,000 people, half of them are vaccinated, which is about 15,000 data volume. Unfortunately, there are only 546 elderly people, very few.
How many people are used to analyze the effectiveness? There were 13,765 people, and there were only 209 people over 60. Note that the effectiveness of the analysis here refers to how much the vaccine really reduces the risk of infection. This is the effectiveness we care about. Don’t be confused by some people using data such as antibody conversion rates.
How effective is the vaccine of SINOPHARM?
SINOPHARM actually has two different vaccines, one is SINOPHARM Beijing and the other is SINOPHARM Wuhan. The effectiveness and safety of different vaccines must be evaluated separately. The effectiveness reported in the media before, SINOPHARM Beijing is 79.3%, SINOPHARM Wuhan is 72.5%. Then the protective effects of moderate to severe cases are 99.5% and 100% respectively.
The WHO evaluated only the vaccine of SINOPHARM Beijing. The effectiveness data comes from the Phase III clinical trial of the clinical trial registration number COVIV-02NCT04510207. The trial sites include the UAE, Bahrain, Egypt and Jordan. The total number of participants is 45,000. But it should be noted that this experiment has both SINOPHARM Beijing and SINOPHARM Wuhan. The WHO should only evaluate the data of SINOPHARM Beijing.
What is the result of SINOPHARM Beijing? The table below is clear.
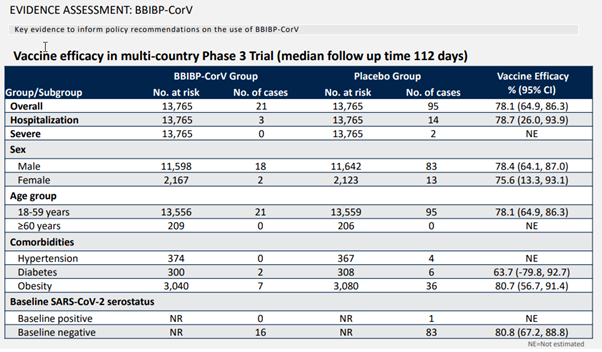
As mentioned before, 13,765 people were vaccinated, corresponding to the same number of placebo groups. These people have been following an average of 112 days. If you look at all confirmed cases of new coronavirus disease, there are 21 cases in the vaccine group and 95 cases in the placebo group. The effectiveness is calculated to be 78.1%, and the confidence interval is 64.9%-86.3%.
The confidence interval was mentioned slightly in the previous article. Since this is really important but has been overlooked by various vaccine effectiveness reports, I repeat it here. SINOPHARM’s test is 78.1% effective this time. Is this effective performance repeated? Will it be this number if you do it again? The confidence interval can be understood as repeated countless times of such clinical trials, and most of the results will be within this range. For SINOPHARM, it is 64.9% to 86.3%. This data can tell us how reliable SINOPHARM’s 78.1% effectiveness is.
These are all COVID-19 cases, so what is the effect on critical illness protection? There are two types of severe illnesses in the trial. One type requires hospitalization and the other type is more serious. The specific criteria are not stated. I guess the difference is probably whether or not oxygen is required.
For hospitalized cases, 3 cases received the SINOPHARM vaccine and 14 cases received a placebo, corresponding to an effectiveness of 78.7% with a confidence interval of 26%-93.9%. From the range of the confidence interval, you can also see that due to the small number of severe cases, the error range of the assessment is large. For more severe cases, then there are only two cases in the placebo group. It should be noted that since this is too small to evaluate the effectiveness alone, it cannot be said that 100% protection is the most severe.
In general, the overall effectiveness of the SINOPHARM vaccine is good. However, due to the trial itself, the amount of data on critical illness protection is limited, and too many conclusions cannot be drawn. As for the 79.3% overall effectiveness previously announced in the media and 99.5% of the protection effectiveness for moderately severe cases, it may be that the data was so at the time, and now it has been updated over time, or some people have other unique algorithms.
Is the effectiveness and safety of SINOPHARM vaccine universal?
In addition to the overall effectiveness and the effectiveness of critical illness protection, in fact, we should be more concerned about whether these effectiveness and safety data can be extended to all people.
For example, if I have an underlying disease, will this vaccine still be effective for me? Is it still safe? If I am older, can this vaccine still be used?
This is why many vaccines emphasize the recruitment of diverse subjects when doing Phase III clinical trials. You know, the most dangerous people after contracting the COVID-19 are the elderly and those with underlying diseases. It is very important that these people participate in clinical trials and obtain relevant effectiveness and safety data.
At this point, the results of the Brazil Phase III clinical trial announced by KEXING are very flawed. There are only more than 400 elderly people over 60 years old, and it is impossible to judge the effectiveness and safety of this age group. Of course, this is the inevitable result of KEXING’s decision to recruit medical staff for the experiment.
Unfortunately, SINOPHARM has not improved at this point. Only 209 people over the age of 60 were vaccinated, corresponding to the placebo group of 206 people. These 415 people were not infected, and it was naturally impossible to evaluate the effectiveness.
In terms of basic diseases, only hypertension, diabetes and obesity are listed. In addition to obesity, more than 3,000 volunteers were vaccinated, which corresponds to an 80.7% effectiveness. The other two basic diseases cannot be analyzed because of the small number of people.
In general, the current clinical trial data cannot explain that the SINOPHARM vaccine is safe and effective in the elderly and patients with underlying diseases. What’s more surprising is that among the volunteers enrolled in SINOPHARM, women accounted for only about 15% (only 2167 women out of 13,765 vaccinators). Although the customs in the Middle East may be a big resistance to recruiting female subjects, it is really necessary to consider other places to do experiments to remedy this. We shouldn’t just develop a vaccine for a bunch of young guys, right?
Other data and unfinished things
The safety of the SINOPHARM vaccine in clinical trials is still good. Although the WHO did not elaborate on this list, it also provided some summary. For example, common adverse reactions are relatively mild, mainly pain at the injection point, headache and fatigue. The total number of serious adverse reactions did not differ significantly between the vaccine group and the placebo group.
However, after all, there are fewer elderly people and patients with underlying diseases in the number of SINOPHARM vaccines. These shortcomings greatly limit the practical significance of the label “good safety”.
SINOPHARM is also trying to make up for it through other research. For example, in China, 1.1 million doses of SINOPHARM vaccine have been vaccinated for people over 60 years old, and 45 cases of adverse reactions are considered to be related to the vaccine. According to this data, the safety of people over 60 years old is still good. However, it should be noted that this kind of post-marketing tracking is greatly affected by the completeness of the local drug safety tracking system. If it is a way of passively relying on the report of the vaccinator, it is easy to underreport. There are not too many details in the WHO document. Out of a responsible attitude to the vaccinators, SINOPHARM needs to make more detailed disclosures of this type of research.
In addition, according to data collected from a post-marketing COVID-19 case in Bahrain (the table below), SINOPHARM is 91% effective over 60 years of age, which is similar to 90% of all adults from the same data source. However, the number of people in this study is very small and there are not too many details. It only said that it was the PCR test results in the Bahrain National Health System during the same time period. There are less than 8,000 adults in it (it seems that this includes the elderly, because the table only says that they are over 18 years old), but there are more than 2,400 positive cases for COVID-19. Such a high positive test rate makes people doubt the data screening process. . There are fewer than 800 people over 60.
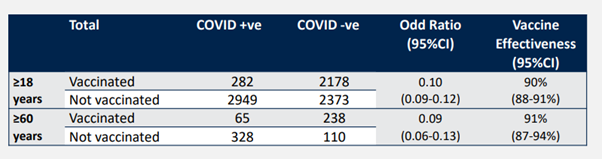
When using such real-world test reports to calculate the effectiveness of vaccines, one must consider whether there is a possibility that vaccinators are less likely to be tested. A person who has been vaccinated, even if infected with symptoms, may feel that it is impossible to get COVID-19 and will not be tested. Therefore, this type of research is to set very detailed data selection standards, and often requires a large amount of raw data. The WHO only released this form, which can be said to be unconvincing.
In addition to the missing data for the elderly and patients with underlying diseases, we do not know whether SINOPHARM is still effective against the constantly emerging mutant virus strains. The WHO should have quoted the results of the SINOPHARM and KEXING serum neutralization experiments previously published in the New England Journal of Medicine, and only said that the neutralization ability of the South African mutant strain was reduced. This aspect certainly needs more research in the future.
Some obvious evidence is missing. WHO has specially summarized a page of slides, including how the SINOPHARM vaccine protects against severe illness, how long the protection is effective, whether it is safe for pregnant women, and whether it is safe and effective for the elderly and people with underlying diseases. These are not difficult to see from our above analysis, and they are all very reasonable. In addition, it also specifically mentioned that the current post-market security tracking data is not enough.
Based on the existing effectiveness and safety data, as well as the “deficiencies” of this kind of evidence, the WHO has also given several overall evaluations of effectiveness and safety, which is the following table.
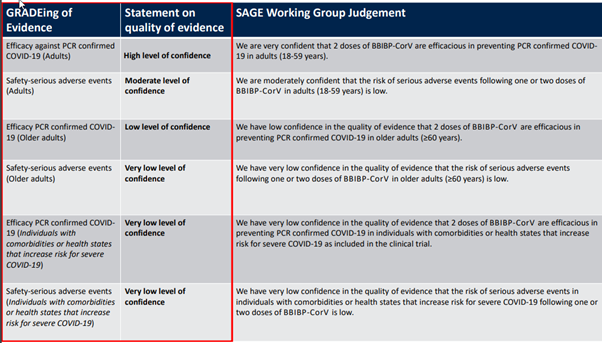
Simply put, for people 18-59 years old, the effectiveness of the protection of the COVID-19 is currently fully evidenced (very confident), and the safety evidence is also acceptable (relatively confident). However, confidence in the effectiveness of the elderly over 60 years old is relatively low. As for the safety in the elderly, the effectiveness and safety in people with underlying diseases, that is very low confidence. These evaluations can be said to be completely based on the existing evidence, whether it is the parts that make people feel comfortable or uncomfortable, they are considered objective and fair.
Now that the global COVID-19 epidemic is serious, the SINOPHARM vaccine, as a vaccine that can protect against the COVID-19, of course can and should be an important tool for the global fight against the epidemic.
But looking back at the various propaganda before this vaccine and the data that has barely been made public now, it is inevitable that people are mixed. From the high rate of 97% antibody conversion as effective, to the emergency authorization to vaccinate millions of people, no one is infected and therefore 100% effective. Such absurd rhetoric comes from the pharmaceutical company itself, which is really embarrassing. After the listing, the Phase III clinical trial data has not been disclosed for a long time. At first glance, both the elderly and patients with underlying diseases are seriously under-recruited. The 99.5% protection against severe illnesses mentioned earlier is also unrecognizable.
Of course, it is impossible for any clinical trial to be comprehensive, and unsatisfactory results are common. But just like people can be poor, but not short-spirited, so can pharmaceutical companies. You can test and fail-this is normal in the high-risk new drug R&D industry, but you must not lose your ambition. The ambition of pharmaceutical companies is not only the courage to try, but also the courage and objective narrative determination of openness and transparency.
China’s pharmaceutical industry is still very young, and there are still many areas to improve, but the premise for improvement is that we first raise the standard, and we can’t just look at our own baby. If you get a score of 60, you can count it as a 100. Fortunately, SINOPHARM has also arranged many follow-up trials and follow-ups, including Phase III clinical trials in Peru and Argentina, which may prove whether the SINOPHARM vaccine is effective against the Brazilian mutant strains that are popular in South America. In China, more safety tracking for the elderly and patients with underlying diseases has also been set up-not only passive collection of data, but also active tracking of vaccinators. This should also resolve some doubts about safety.
I hope that all Chinese vaccines, including SINOPHARM and KEXING, can enter the Chinese people’s vaccination plan in a more open, transparent, rigorous and realistic manner, and come to the forefront of international anti-epidemic!
China SINOPHARM COVID-19 Vaccine is not safe for Seniors or Women ?
(source: internet, kepuxiaoyuan, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



