Bayer New drug Finerenone for diabetic nephropathy!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Bayer New drug Finerenone for diabetic nephropathy!
Bayer New drug Finerenone for diabetic nephropathy! New medicine for diabetic nephropathy! Bayer’s first drug, finerenone, significantly reduces the risk of new-onset atrial fibrillation or flutter.
Bayer recently announced the latest data from a pre-specified exploratory analysis in the Phase 3 FIDELIO-DKD study of a new diabetic nephropathy drug finerenone for the treatment of chronic kidney disease (CKD) patients with type 2 diabetes (T2D).
The results show that when combined with standard care, finerenone can significantly reduce the risk of new atrial fibrillation or flutter (AFF) in patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) compared with placebo. The analysis also showed that compared with placebo, finerenone reduced the risk of composite primary endpoints and composite key secondary endpoints, and there was no significant difference in efficacy between patients with and without a history of AFF at baseline.
These data have been announced at the 70th Annual Scientific Meeting of the American College of Cardiology (ACC.21) held recently, and were published in the Journal of the American College of Cardiology (JACC) at the same time. The title of the article is: Finerenone Reduces Onset of Atrial Fibrillation. in Patients with Chronic Kidney Disease and Type 2 Diabetes.
Finerenone is a first-of-its-kind, non-steroidal, selective mineralocorticoid receptor antagonist (MRA), which has been proven to have positive renal and cardiovascular benefits in patients with CKD and T2D in the phase III FIDELIO-DKD study . Based on the research data, in mid-November 2020, Bayer simultaneously submitted finerenone’s marketing application documents to the US FDA and the EU EMA. In January 2021, the US FDA accepted finerenone’s new drug application and granted priority review. In February 2021, Bayer submitted a new drug marketing application for finerenone in China for the treatment of patients with CKD and T2D.
Based on a comprehensive clinical trial project, finerenone is the first non-steroidal selective mineralocorticoid receptor antagonist (MRA) that has been proven to have cardiovascular and renal benefits for patients with CKD and T2D. Although progress has been made in recent years, many patients with CKD and T2D are still moving towards end-stage renal failure or premature death. The mechanism of action of finerenone is different from current treatments. If approved, the drug can slow the progression of the disease by directly targeting inflammation and fibrosis (the main drivers of CKD progression).
The FIDELIO-DKD study was carried out in patients with CKD and T2D to evaluate the efficacy and safety of finerenone and placebo. Both groups received standard care, including hypoglycemic therapy and maximum tolerated dose of renin-angiotensin system (RAS) blockade therapy, such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptors Blocker (ARB).
The exploratory analysis results announced at this meeting showed that compared with the placebo group, the prevalence of AFF in the finerenone group was lower (respectively: 120 cases [4.2%] vs 153 cases [5.4%]). Among patients without a history of AFF, the incidence of new AFF in the fennidone group was significantly lower than that in the placebo group (respectively: 82/2593 [3.2%] vs 117/2620 [4.5%]; incidence per 100 patients per year Rate: 1.20 and 1.72 respectively; HR=0.71; 95%CI: 0.53-0.94; p=0.0164).
In terms of the effect of finerenone on the overall incidence of major renal outcomes (time to renal failure, continuous reduction of eGFR from baseline ≥40%, and renal death compound), the effect of fineenone between patients with and without AFF history There was no significant difference (HR=1.13, 95%CI: 0.71-1.79; HR=0.81, 95%CI: 0.71-0.91; interaction p-value=0.16). For key secondary endpoints (combination of time spent in hospitalization for cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and heart failure compared with placebo), the efficacy of finerenone in patients with and without a history of AFF is Consistent (HR=0.88; 95%CI: 0.62-1.24; HR=0.85, 95%CI: 0.73-0.99; interaction p-value=0.85).
Gerasimos Filippatos, MD, Ph.D., co-lead investigator of the FIDELIO-DKD study and professor of cardiology at the National and Capoditri University of Athens, Greece, said: “Patients with chronic kidney disease and type 2 diabetes have a significantly increased risk of atrial fibrillation This is an increasingly serious health problem that increases the risk of heart failure and stroke risk by 5 times. This exploratory analysis deepens our understanding of finerenone by reducing new atrial fibrillation in patients with chronic kidney disease and type 2 diabetes Or flutter risk to understand the potential ability to resolve cardiovascular outcomes.”
Dr. Christian Rommel, Member of the Executive Committee of Bayer Pharmaceuticals and Director of R&D, said: “The unfortunate reality is that patients with chronic kidney disease and type 2 diabetes are often characterized by complex risk conditions, and the risk of cardiovascular events is that of patients with simple type 2 diabetes. 3 times. We are very pleased to see the potential benefits of finerenone in reducing the risk of cardiovascular events in this underserved patient population. These results complement the existing evidence of finerenone as they illustrate how this potential new treatment option is Address the unmet needs of cardiovascular and renal care while improving the prognosis of patients.”
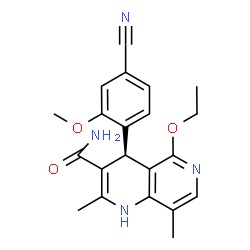
The chemical structure of finerenone (picture source: newdrugapprovals.org)
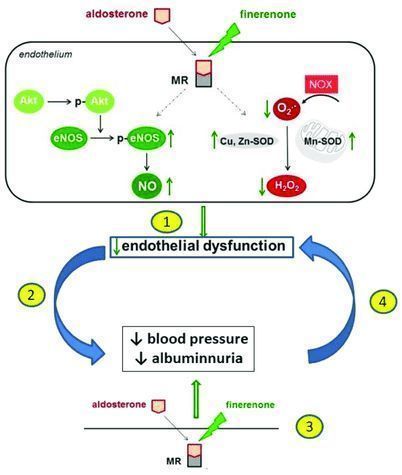
Finerenone (BAY 94-8862) is an investigational, non-steroidal, selective mineralocorticoid receptor antagonist (MRA) that has been shown to reduce the harmful effects of excessive mineralocorticoid receptor (MR) activation. Excessive activation of mineralocorticoid receptors is the main driver of kidney and heart damage. In 2015, the US FDA granted Finerenone Fast Track Status (FTD).
Chronic kidney disease (CKD) is one of the most common complications of diabetes and an independent risk factor for cardiovascular disease. In all patients with type 2 diabetes, approximately 40% of patients will develop CKD. CKD is the main cause of end-stage renal disease and renal failure. In the advanced stage, patients may require dialysis or kidney transplantation to survive. In 10 years, type 2 diabetes patients with CKD are three times more likely to die from cardiovascular-related diseases than patients with type 2 diabetes alone. It is well known that in patients with CKD and type 2 diabetes, excessive activation of mineralocorticoid receptors can trigger harmful processes (for example, inflammation and fibrosis) in the kidneys and heart. Globally, CKD in patients with type 2 diabetes is the most common cause of renal failure.
The mechanism of finerenone (picture source: researchgate.net)
The finerenone phase III clinical project is by far the largest CKD phase III clinical project. The project consists of 2 studies and enrolled 13,000 T2D patients with widespread CKD disease from all over the world, including patients with early renal damage and more advanced renal disease. This project aims to evaluate the effects of finerenone and placebo in combination with standard care on the prognosis of kidney and cardiovascular (CV).
FIDELIO-DKD (finerenone reduces renal failure and disease progression in diabetic nephropathy) is a randomized, double-blind, placebo-controlled, parallel-group, multi-center, event-driven phase III study, enrolling more than 1,000 from 48 countries around the world Approximately 5700 T2D patients with CKD at the site. In the study, these patients were randomly assigned to receive 10 mg or 20 mg of finerenone or placebo once a day, while receiving standard care, including hypoglycemic therapy and maximum tolerated doses of renin-angiotensin system (RAS) blockers , Such as angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB). The study has reached its primary endpoint.
The FIGARO-DKD (finerenone reduces the incidence and death of cardiovascular disease in diabetic nephropathy) is still in progress. The study enrolled about 7,400 T2D patients with CKD in 48 countries including Europe, Japan, China, and the United States. To investigate the efficacy and safety of finerenone and placebo combined with standard care in reducing the incidence and death of CV. The results announced on May 9 this year showed that the study reached the primary endpoint: when combined with standard care, finerenone significantly reduced the first cardiovascular (CV) death or non-fatal CV event (myocardial infarction) compared with placebo. , Stroke, hospitalization for heart failure).
Bayer recently announced the launch of the FINEARTS-HF study, a multicenter, randomized, double-blind, placebo-controlled Phase III study that will examine more than 5,500 patients with symptomatic heart failure (HF) with a left ventricular ejection fraction ≥40% (New York Heart Association Class II-IV) Finerenone and placebo were investigated. The main purpose of the study is to prove that finerenone is superior to placebo in reducing the incidence of the composite endpoint of CV death and overall (first and recurring) HF events (defined as heart failure or emergency HF visits).
(source:internet, reference only)
Disclaimer of medicaltrend.org



