New psychiatric drug: BXCL501 is under review in United States!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New psychiatric drug: BXCL501 is under review in United States!
New psychiatric drug: BXCL501 is under review in United States! New psychiatric medicine! Significant progress in ten years of radical acute treatment: BXCL501 (dexmedetomidine sublingual film) is under review in the United States!
BioXcel Therapeutics (BTI) is a clinical-stage biopharmaceutical company focused on using new artificial intelligence methods to develop transformative drugs in the fields of neuroscience and immuno-oncology.
Recently, the company announced that the U.S. Food and Drug Administration (FDA) has accepted a new drug application (NDA) for BXCL501 (dexmedetomidine sublingual film), which is used for schizophrenia and bipolar disorder I Acute treatment of type and type II related agitation.

According to the “Prescription Drug User Fees Act” (PDUFA), the target date for the FDA to make a resolution on the NDA is January 5, 2022. Currently, the FDA does not plan to convene an advisory committee meeting to discuss the application. If approved, BXCL501 will represent the first major advancement in the acute treatment of schizophrenia and bipolar disorder-related agitation in the past decade.
BXCL501 is an under-research, proprietary, orally dissolved dexmedetomidine (dexmedetomidine) film preparation; dexmedetomidine is a selective α2a receptor agonist, clinically used as an intravenous preparation, For the treatment of agitation and opioid withdrawal symptoms.
BioXcel believes that BXCL501 potentially targets a causal agitation mechanism. Anti-agitation effects have been observed in multiple clinical studies of various neuropsychiatric diseases, including schizophrenia-related agitation (SERENITY I) and bipolar disorder Related agitation (SERENITY II), dementia related agitation (TRANQUILITY).
Previously, the US FDA has granted BXCL501 a breakthrough drug designation (BTD) for acute treatment of dementia-related agitation, and fast track designation (FTD) for acute treatment of agitation related to schizophrenia, bipolar disorder and dementia.
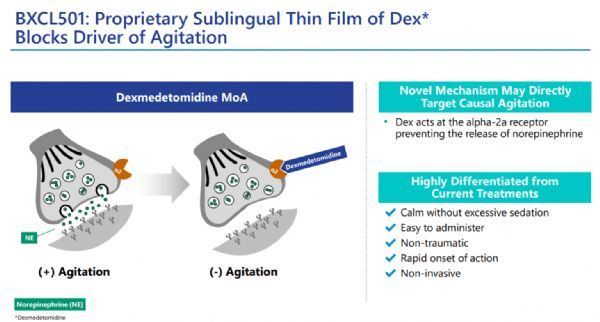
BXCL501 mechanism of action
Agitation is a common and difficult to control symptom associated with a variety of neuropsychiatric diseases, including schizophrenia and bipolar disorder type I and type II. At present, agitation is still a growing medical burden. It is estimated that these two diseases alone have about 9 million adult patients in the United States, and more than 3 million people experience agitation each year. On average, patients with these diseases experience more than a dozen seizures each year, most of which require medication.
Early recognition and timely intervention to reduce agitation are essential to avoid the escalation of symptoms and the appearance of aggression. Expert consensus best practice guidelines suggest that agitation should be combined with behavioral sedation techniques, speech relaxation, and medications voluntarily accepted by the patient, without being forced, and the goal of pharmacology is “sedation but not excessive sedation.” A non-invasive treatment that can provide rapid and sustained relief of symptoms may help avoid expensive and traumatic coercive measures such as physical restraint and isolation, which can lead to hospital admissions and prolonged stays.
BXCL501 NDA is supported by data from 2 randomized, double-blind, placebo-controlled, parallel-group Phase 3 studies (SERENITY I and SERENITY II). These two studies separately evaluated BXCL501 for the acute treatment of schizophrenia and bipolar disorder type I and type II related agitation. In these two studies, BXCL501 was well tolerated and reached the primary and secondary endpoints at both 120mcg and 180mcg doses, indicating that in multiple agitation scales, compared with placebo, it was statistically significant and compared with the baseline level. Fast and lasting improvement.
Vimal Mehta, CEO of BioXcel, said: “The submission of the NDA marks an important milestone in our goal of providing new treatment options for millions of patients with schizophrenia and bipolar disorder. We believe that if BXCL501 is approved, it will It represents a significant improvement in the aggressive care and management of these patients, and has the potential to reduce the burden on doctors and related caregivers. While the FDA is reviewing our NDA, we will continue to implement our comprehensive business strategy to ensure that we can do well Bring BXCL501 to patients and healthcare providers across the United States to address important unmet medical needs in the field of radical therapy.”(
(source:internet, reference only)
Disclaimer of medicaltrend.org



