FDA approved Denmark long-acting growth hormone: Skytrofa
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approved Denmark long-acting growth hormone: Skytrofa
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- The viral load of Delta variant is 1260 times the original COVID-19 strain
FDA approved Denmark long-acting growth hormone: Skytrofa.
New medicine for growth hormone deficiency in children! U.S. FDA approves long-acting growth hormone Skytrofa (lonapegsomatropin-tcgd). Skytrofa is the first unmodified long-acting (once a week) growth hormone
The Danish biopharmaceutical company Ascendis Pharma recently announced that the US Food and Drug Administration (FDA) has approved the weekly long-acting growth hormone Skytrofa (lonapegsomatropin-tcgd, TransCon hGH) for the treatment of growth hormone deficiency in children GHD, specifically: pediatric patients aged 1 year and over, weighing at least 11.5 kg, and growth failure due to insufficient secretion of endogenous growth hormone (GH). VISEN Pharmaceuticals has the exclusive license of Skytrofa in Greater China and is currently conducting Phase 3 clinical trials of the product.
Skytrofa is the first long-acting growth hormone product to treat children’s GHD. It is injected once a week. This drug is the first product approved by the FDA to release somatropin (growth hormone) continuously within a week, and it is also the first product approved by the FDA. A product that uses TransCon technology.
Skytrofa is a long-acting prodrug of human growth hormone (hGH). It is administered once a week and it releases the same growth hormone (somatropin) as the growth hormone in the once-daily growth hormone product.
Clinical data shows that, compared with once-a-day growth hormone products, once-a-week Skytrofa exhibits a higher annualized growth rate (AHV) in week 52, and has similar safety and tolerability.
Growth hormone deficiency (GHD) is a serious and rare disease characterized by short stature and metabolic complications. In GHD, the pituitary gland cannot produce sufficient growth hormone, which is not only important for height, but also for the child’s overall endocrine health and development.
The approval includes the new Skytrofa® auto-injector and cartridges, which allow families to store medications at room temperature for up to 6 months after being removed from the refrigerator for the first time. By shifting to once-a-week injections, patients who previously injected once a day are expected to reduce the number of injection days per year by 86%.
In terms of new GHD drugs, in September 2020, Novo Nordisk’s once-a-week long-acting growth hormone derivative Sogroya (somapacitan-beco) was approved by the US FDA for the treatment of GHD in adults. Sogroya is the first human growth hormone (hGH) therapy for the treatment of adult GHD that requires only subcutaneous injections once a week, while other FDA-approved hGH preparations must be injected daily.
Sogroya is modified from natural hGH to enhance its binding to the plasma protein albumin (albumin), making it suitable for once-a-week dosing. Currently, Sogroya is also developing a treatment for GHD in children.
Skytrofa (TransCon hGH) is the world’s only human growth hormone prodrug designed with the patented “Transient Conjugation” technology. The drug’s mechanism of action is different from other technology’s long-acting growth hormone analogs. It can ensure the release of unmodified and active human growth hormone in the human body for 7 days, ensuring that the tissue distribution of active human growth hormone in the body is consistent with the once-daily recombinant growth hormone (rhGH). In the United States and Europe, TransCon hGH has been granted Orphan Drug Designation (ODD) for the treatment of GHD.
Childhood growth hormone deficiency (GHD) is a serious and rare disease caused by insufficient growth hormone secreted by the pituitary gland. Children with GHD are not only short in stature, but also have metabolic abnormalities, psychosocial challenges, cognitive deficits, and poor quality of life. For decades, the standard of care for GHD has been to inject hGH subcutaneously once a day to improve growth and metabolic effects. For caregivers and patients, the treatment burden of daily injections is high, which may lead to poor compliance and reduce the overall treatment effect.
heiGHt research results
The FDA approved Skytrofa for the treatment of GHD in children, based on the results of the international phase 3 heiGHt study. This is a randomized, open-label, positive drug controlled study in 161 newly-treated children with GHD, comparing Skytrofa once a week with a somatropin product (GenotropinGHD) once a day.
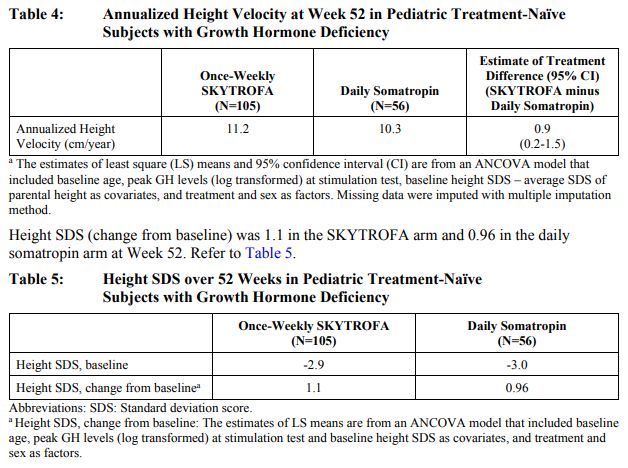
The results showed that the study reached the primary endpoint: at week 52, Skytrofa was not inferior to daily hGH and better than daily hGH in terms of annual growth rate (AHV, unit: cm/year), and had similar safety sex. The specific data is that using the analysis of covariance (ANCOVA) intention-to-treat group, the AHV in the Skytrofa treatment group was 11.2 cm/year, and the daily hGH group was 10.3 cm/year (the difference between the two groups: 0.9 cm/year, 95% CI: 0.2-1.50).
It is worth mentioning that at each follow-up, the AHV of the Skytrofa treatment group was higher than that of the daily hGH treatment group, and the treatment difference reached statistical significance from the 26th week (including the 26th week). The proportion of patients with poor response (AHV<8.0 cm/year) in the Skytrofa treatment group and daily hGH treatment group was 4% and 11%, respectively. All the sensitivity analyses completed in the study support the main results, demonstrating the robustness of these results.
TransCon technology
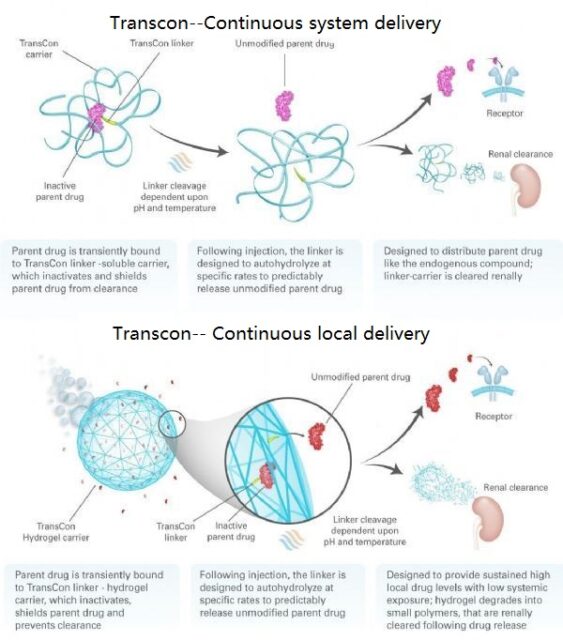
TransCon means “Transient Conjugation”. Ascendis’ proprietary TransCon platform is an innovative technology designed to create new therapies that optimize therapeutic effects, including efficacy, safety, and dosing frequency. The TransCon molecule has three components: an unmodified parent drug, an inert carrier to protect it, and a linker that temporarily connects the two. When connected, the carrier is inactivated and protects the parent drug from being eliminated. When injected into the body, physiological pH and temperature conditions will begin to release the unmodified active parent drug in a predictable release mode. Because the parent drug is unmodified, its original mode of action should remain unchanged. TransCon technology can be widely used in multiple therapeutic areas of proteins, peptides or small molecules, and can be used systemically or locally.
Currently, Ascendis is using TransCon technology to establish a leading, fully integrated rare disease company. The company uses TransCon technology and clinically proven parent drugs to create new therapies that have best-in-class efficacy, safety and/or convenience. In addition to children’s GHD, the company is also developing Skytrofa (TransCon hGH) for the treatment of adult GHD in Phase III clinical development. In addition, the company has 2 rare endocrinology assets in Phase II clinical trials: (1) TransCon PTH is a long-acting parathyroid hormone (PTH) prodrug used to treat hypoparathyroidism; (2) TransCon CNP is a long-acting C-type natriuretic peptide prodrug used to treat achondroplasia and other FGFR-related bone diseases.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



