Long-acting antihypertensive drug Zilebesiran: One injection for half a year
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Long-acting antihypertensive drug Zilebesiran: One injection for half a year
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Long-acting antihypertensive drug Zilebesiran: One injection for half a year.
NEJM: One injection for half a year! Long-acting antihypertensive drug Zilebesiran: Clinical phase 1 study data released, antihypertensive effect can be stable for 24 weeks.
Long-term blood pressure lowering, half a year at a time?
On July 20, the “New England Journal of Medicine” published a new result of a clinical study.
The RNA interference drug Zilebesiran showed good safety and dose-dependent antihypertensive effect in a phase 1 clinical study, and a single administration can achieve continuous antihypertensive effect for up to 24 weeks .
 Thesis title map
Thesis title map
Zilebesiran is an RNA interference drug consisting of a small interfering RNA (siRNA) covalently linked to an N-acetylgalactosamine (GalNac) ligand that specifically reduces hepatic angiotensinogen mRNA levels, thereby reducing angiotensinogen levels .
The renin-angiotensin-aldosterone system (RAAS) plays an important role in blood pressure regulation. Angiotensinogen is the only precursor of all angiotensins, and inhibition of RAAS by Zilebesiran could theoretically limit compensatory angiotensin activation by angiotensin-converting enzyme inhibition or angiotensin receptor blockers.
In addition, GalNac-siRNAs have long-lasting pharmacodynamic effects, which can provide blood pressure lowering effects lasting for 24 hours or even several months, which makes subcutaneous administration possible twice a year or quarterly .
In this 4-part, multicenter Phase 1 study, researchers attempted to explore the safety, pharmacokinetics and pharmacodynamics of Zilebesiran.
Part A and Part B are randomized double-blind placebo-controlled trials, Part A is administered with gradient doses, Part B is a fixed dose under low-salt/high-salt diet conditions; Part E is an open-label study, Zilebesira combined with irbesartan; Part D is still in progress; Part C has been cancelled.
The study participants were hypertensive patients aged 18-65, with sitting systolic blood pressure of 130-165mm Hg (A/B) or 135-165mm Hg (E), and 24-hour ambulatory blood pressure monitoring after stopping antihypertensive drugs for two weeks. The average systolic blood pressure exceeded 130mm Hg.
Participants with secondary hypertension, orthostatic hypotension, diabetes, previous cardiovascular events, and those requiring β-adrenoceptor blockade were excluded from the study.
In Part A, patients received a single injection of Zilebesiran (10/25/50/100/200/400/800 mg) or placebo in a 2:1 ratio. Antihypertensive drugs were allowed for those whose blood pressure was still uncontrolled in the 8th week.
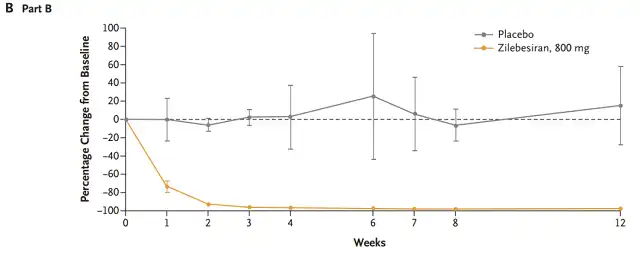
In part B, from day 21 to day 8 of the trial, participants received a low-salt diet (0.23g/day) and a high-salt diet (5.75g/day) sequentially, followed by a 2:1 allocation to 800mg Zilebesiran and placebo, and repeated the diet program on days 43-56 (the expected peak time of Zilebesiran’s effect) to evaluate the impact of dietary salt intake on the antihypertensive effect of Zilebesiran.
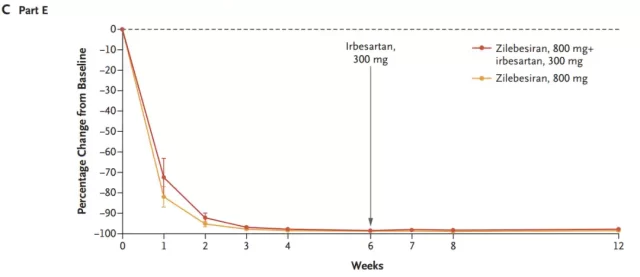
In part E, all participants received 800 mg of Zilebesiran, and those whose systolic blood pressure exceeded 120 mm Hg in 24-hour ambulatory blood pressure monitoring at week 6 received additional treatment with irbesartan, 300 mg per day, for 2 weeks.
All trials ended at the 12th week and entered follow-up. Participants were instructed to drink alcohol appropriately during the trials, avoid taking supplements that affect blood pressure, and control daily salt intake to 2g except for part B restrictions.
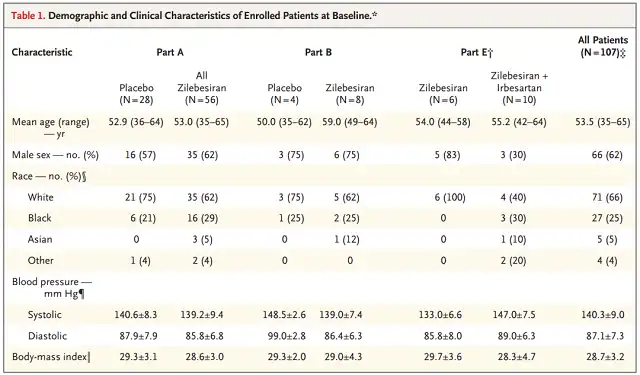 Participant baseline condition
Participant baseline condition
Adverse events were reported in 72% of zilebesiran-treated patients and 88% of placebo-treated patients, with injection site reactions being the only treatment-related adverse reactions reported in more than 2 patients. There were no reports of hypotension, hyperkalemia, or worsening renal function leading to medical intervention.
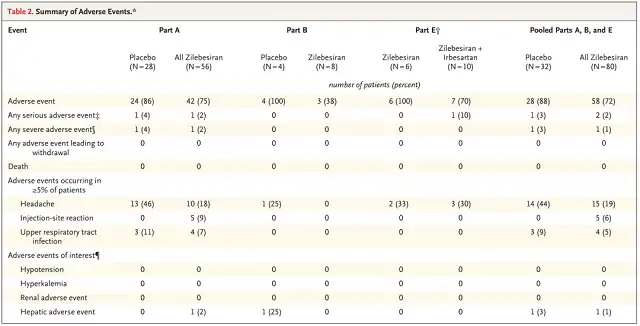 Adverse event
Adverse event
The pharmacokinetics of Zilebesiran exhibited a clear dose profile. In part A, the change in serum angiotensinogen level was negatively correlated with the dose of Zilebesiran at week 8. In the 800mg group, the serum angiotensinogen level decreased by more than 90% within one week, and similar performance was also observed in the B/E part .
Results in Part E do not support an additional effect of irbesartan on angiotensinogen levels. Small decreases in plasma renin activity and aldosterone and angiotensin 1/2 levels were observed in 200 mg and higher dose groups.
Three sets of test results
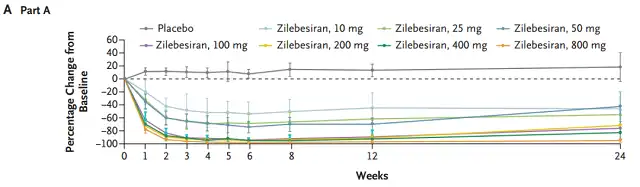
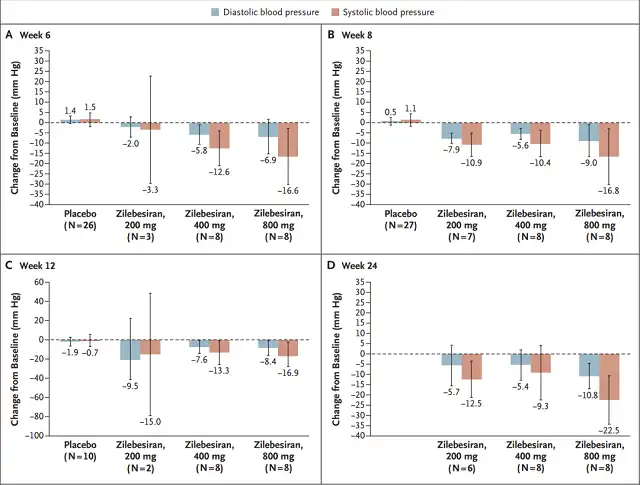
In part A, it was observed that Zilebesiran treatment was negatively correlated with the change in 24-hour dynamic mean systolic blood pressure , and the degree of reduction in systolic blood pressure correlated with the degree of reduction in angiotensinogen. It can be seen that the antihypertensive effect lasted until the 24th week.
At 24 weeks, mean reductions in systolic and diastolic blood pressure were 22.5±5.1mm Hg and 10.8±2.7mmHg in the 800mg group.
 Part A Zilebesiran shows long-term blood pressure-lowering effects
Part A Zilebesiran shows long-term blood pressure-lowering effects
In part B, the low-salt diet one week before administration reduced the mean systolic and diastolic blood pressure by 9.1±4.5mm Hg and 2.4±3.1mm Hg, respectively, and returned to baseline levels after the high-salt diet.
After administration, systolic and diastolic blood pressure decreased by 18.8 ± 4.3 mm Hg and 8.4 ± 2.5 mm Hg, respectively, after a low-salt diet, and returned to baseline levels again after a high-salt diet.
In Part E, patients who received only Zilebesiran had a reduction in systolic blood pressure of 21.8 ± 2.9 mm Hg from baseline at week 6. Patients who received additional treatment with irbesartan had a mean reduction in systolic and diastolic blood pressure of 6.3 ± 3.1 mm Hg and 3 ± 1.9 mm Hg at week 8.
It can be seen that the antihypertensive effect of Zilebesiran is really worth looking forward to, but this is only a phase 1 study after all, the scale and duration are not enough, and follow-up studies are needed for detailed discussion.
References:
[1] https://www.nejm.org/doi/full/10.1056/NEJMoa2208391
Long-acting antihypertensive drug Zilebesiran: One injection for half a year
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



