NEJM: ADC drugs significant trial results for non-small cell lung cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
NEJM: ADC drugs significant trial results for non-small cell lung cancer
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
NEJM: ADC drugs significant trial results for non-small cell lung cancer. Clinical trials of ADC drugs in the treatment of non-small cell lung cancer, with significant results.
About 3% of non-small cell lung cancers (NSCLC) have HER2 gene mutations. Non-small cell lung cancers with HER2 gene mutations are more common in people who have never smoked than in people who have smoked. They usually have a poor prognosis and often occur. Brain transfer.
Although HER2 targeted therapy has changed the treatment of breast and gastric cancer patients, HER2 targeted therapy has not yet been approved for non-small cell lung cancer patients. Therefore, such patients are usually treated with standard chemotherapy or immunotherapy, but the effect is not satisfactory.
In addition, only 27% of patients in this population have an objective response to immune checkpoint inhibitors, and most patients respond poorly.
Trastuzumab-drutecan (DS-8201) is an antibody-conjugated drug (ADC) composed of a humanized anti-HER2 monoclonal antibody (Trastuzumab), a cleavable tetrapeptide-based linker and a cytotoxic topology It consists of an isomerase I inhibitor (DXd), which prevents cancer cells from replicating DNA, leading to the death of cancer cells.
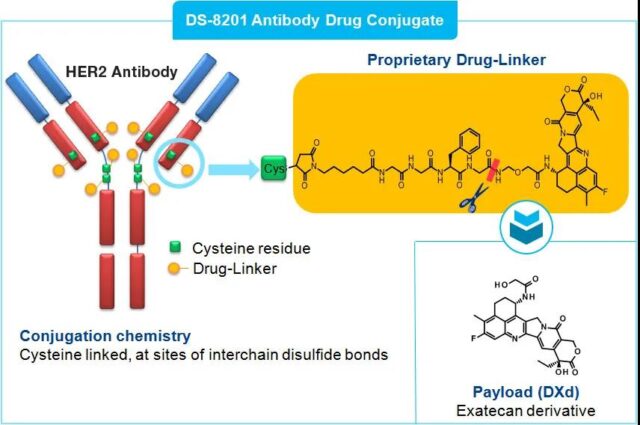
Structure of trastuzumab-drutecan (DS-8201)
Trastuzumab-drutecan has been approved for the treatment of patients with HER2-positive metastatic breast and gastric cancer. So is the ADC drug equally effective against HER2 mutated non-small cell lung cancer?
On September 18, 2021, researchers from the Dana-Farber Cancer Institute in the United States published a titled: Trastuzumab Deruxtecan in HER2-Mutant Non-Small in the New England Journal of Medicine (NEJM), the first of the four major international medical journals -Research paper on Cell Lung Cancer.
In this multi-center international trial of patients with advanced HER2 mutant non-small cell lung cancer, trastuzumab-drutecan treatment showed a high response rate and prolonged the survival of patients, only 2% Of patients had fatal incidents.
However, 20% of patients develop interstitial lung disease, so careful safety monitoring and management are required. In conclusion, this study provides direct evidence for the long-lasting anti-cancer activity of ADC drugs in such lung cancer patients.
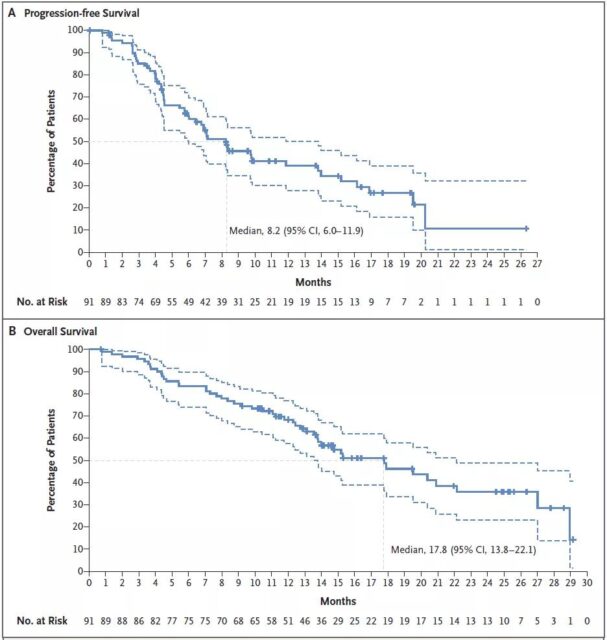
From May 30, 2018 to July 21, 2020, the research team recruited 91 patients with HER2 mutant non-small cell lung cancer and placed them on trastuzumab-drutecan treatment. Among 91 patients, one patient (1%) had a complete remission, 49 patients (54%) had a partial remission, and the vast majority of patients (92%) had tumor shrinkage.
Of the 33 patients with central nervous system metastases at the baseline examination, 14 had received radiotherapy to the brain and 19 had not received radiotherapy. Among these patients, 8 and 10 patients had partial remissions, respectively.
A total of 47 patients (52%) died. The median remission period of 91 patients was 9.3 months, the median progression-free survival was 8.2 months, and the median overall survival was 17.8 months. Among the 33 patients with central nervous system metastases at the baseline examination, the median progression-free survival was 7.1 months, and the median overall survival was 13.8 months.
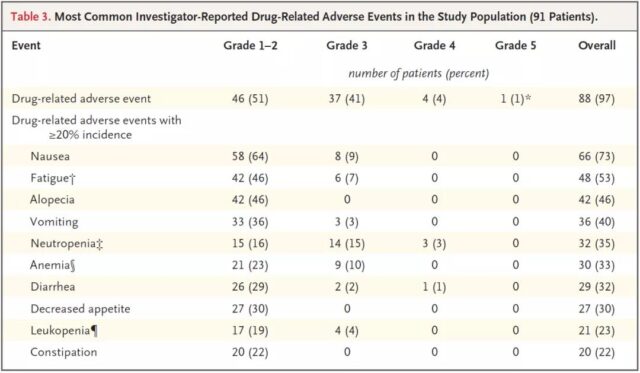
At least one adverse event occurred in 88 patients (97%), and the research team reported that these events were related to trastuzumab-drutecan. 47% of patients had mild adverse events such as gastrointestinal bleeding, loss of appetite, and hair loss, 18 patients (20%) had serious adverse events such as interstitial lung disease, and 2 patients had fatal adverse events.
As a result of treatment, 24 patients developed interstitial lung disease or lung scars. Due to this problem, the research team stopped the treatment of 16 patients and discontinued the treatment of 8 patients. Patients in this condition received steroid therapy.
In summary, these data indicate that the HER2 mutation in non-small cell lung cancer can be used as a therapeutic target, and trastuzumab-drutecan showed long-lasting resistance in 91 patients with HER2 mutant non-small cell lung cancer. Cancer activity: 55% of patients had a proven objective therapeutic effect.
The median duration of the therapeutic effect was 9.3 months, the median progression-free survival was 8.2 months, and the median overall survival was 17.8 months. These results support the use of trastuzumab to treat patients with HER2 mutant non-small cell lung cancer.
The clinical trial was funded by Daiichi Sankyo and AstraZeneca. Trastuzumab-drutecan (DS-8201) was developed by the Japanese pharmaceutical company Daiichi Sankyo. AstraZeneca and Daiichi Sankyo signed a US$6.9 billion global joint development and commercial cooperation in March 2019. Agreement.
Paper link:
https://www.nejm.org/doi/10.1056/NEJMoa2112431
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



