What’s syringe check and how to conduct syringe check?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
What’s syringe check and how to conduct syringe check?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What’s syringe check and how to conduct syringe check?
Syringes are arguably the most difficult container to inspect. Among other obstacles, the syringe requires a unique processing flow, and its shape and size range is wide, requiring customized inspections of multiple areas, which poses obstacles to the accuracy of each project and the overall production speed.
This article explores the various parameters that define syringe inspection, and discusses the best practices for inspecting syringes with different closure types and syringes filled with various drugs, including vaccines and viscous drugs.
Handling of syringe inspection
Although other containers such as vials, cartridges, and ampoules are largely “stationary” and can therefore enter the inspection process independently, syringes usually need to be transported through a conveyor belt and then turned upside down.
In order to inspect syringes accurately and effectively, they usually have to be rotated with their heads upside down, so that any hidden particles in the syringe funnel can sink into the liquid to improve the detectability of the inspection.
If the syringe is sterilized, the container is usually in a “nested barrel” arrangement, which requires it to be unnested by a robotic unit that communicates with the inspection machine.
This complicated process requires gentle and careful handling, and contact between glass and glass should be avoided to reduce the possibility of cracking or breakage.
After entering the inspection turntable, turn the syringe upside down for inspection. The syringe can be fixed to the upper and lower part using a shaft with a custom cup, or the syringe can be fixed under the plunger with a two-finger adaptive clamp.
The method of holding the syringe in the upper and lower parts allows for higher rotation speeds. The syringe with the needle shield can be fixed in this way.
If the diameter of the shield is smaller than the diameter of the syringe body, the rigid needle shield can be fixed. Unfortunately, this processing system is not compatible with all closed systems.
Clamping systems usually run at slower speeds, but are well suited for more sophisticated closure systems, such as Luer locks. The holder does not touch the closure mechanism, so the risk of affecting the integrity of the closure is low.
Inspection process
As mentioned earlier, the inherent multi-component nature of the syringe makes it very complicated to inspect. One of the more challenging parts is the flange ports, because they are usually not completely flat.
This factor often produces deceptive shadows and reflections, both of which make it more difficult for inspection stations to determine whether the glass is scratched or otherwise damaged.
The solution usually lies in the use of appropriate optical and lighting settings, combined with more advanced image processing functions, to minimize false alarms.
For similar reasons, another multi-part component plunger is also an inspection pain point. In fact, plungers can be so tricky that dual control systems are often required to avoid high levels of false rejections. The dual-view optical method allows two spatially coherent views to be analyzed in one image without repeated inspections.
Responsible for checking the angle view of the top of the plunger combined with the front view of the transverse section of the top of the monitoring plunger. Generally speaking, this strategy of combining two complementary views can improve detection performance and reduce false alarm rates.
In Figure 1 below, the area of interest (green outline) is shown in the front view (right) and the tilted top view (left). If an element is detected as a bubble in one view but not another view, as shown in the figure below, there is no need to reject the detection result.
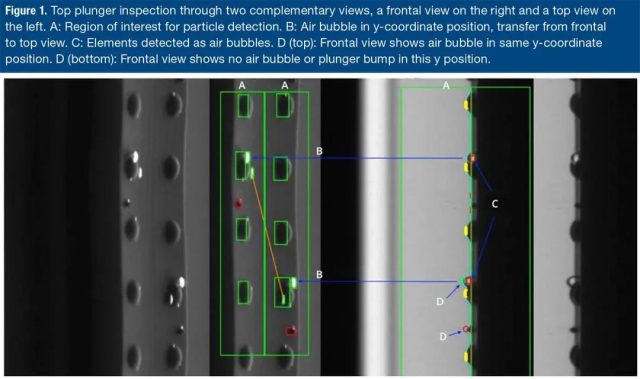 Figure 1. Check the top plunger through two complementary views (right front view and left top view).
Figure 1. Check the top plunger through two complementary views (right front view and left top view).
A: The region of interest for particle detection.
B: The bubble at the y coordinate position, from the front to the top view.
C: Elements detected as bubbles. D (top): The front view shows bubbles with the same y coordinate position.
D (bottom): The front view shows that there is no air bubble or plunger protrusion at this y position.
Since the particles can move in and therefore have limited space to be noticed, the relatively small diameter of the syringe also presents inspection problems.
In order to check injectable drugs, the particles in the formulation may be moved-this process requires the syringe to rotate at speeds as high as 9000 RPM.
The single-rotation device on the turntable spindle meets this need and ensures that the various medicines can be flexibly rotated at the speed most suitable for their personal examination.
However, another question arises here: Although high-speed rotation is sufficient for liquid medicine, what if the container cannot be rotated at high speed for stability reasons?
For example, subjecting many biopharmaceutical drugs to such turbulence may affect their integrity. In addition, for highly viscous drugs, the particles usually do not move, so they cannot be distinguished by this common strategy.
In these scenarios, the answer often lies in three-dimensional inspection, which allows the module to infer whether the suspected contaminant is inside or outside the container by analyzing its trajectory; specifically, if the foreign body is inside, the radius will be shorter, and the outside will be longer. .
Another technique when checking the syringe is to use a line scan camera. When inspecting turbid liquids, a particularly effective method is to use a line scan camera to continuously capture images line by line, and then stitch together the combined images of more than 10,000 exposures.
By rotating the container at high speed, the particles move to the container barrel. The line scan is fast and accurate, resulting in a particle detection rate of almost 100% and a very low false rejection rate.
This setting is very suitable for inspection of cylindrical surfaces, especially when inspecting the appearance of the side, because there is no distortion.
Appropriate lighting conditions are a prerequisite for identifying abnormalities in liquid medicines, because due to the lack of contrast, recognition cannot be performed under natural lighting conditions by the human eye or current automatic visual inspection technology.
The light intensity should be sufficient to illuminate the container while providing moving contrast to identify the smallest particles.
Reliable detection must combine the advantages of various lighting methods to detect the widest range of pollutants, because different pollutants react to light in different ways.
Figure 2 below illustrates the use of back and lateral illumination to detect light-absorbing and reflective particles.

Figure 2. Simultaneous back and side light detection for detecting absorbing particles (left) and reflective particles or fibers (right).
Challenging inspection
Some formulas are more difficult to check than others. For example, in water-like liquids or lightweight suspensions, dynamic trajectory analysis increases the probability of detection because the particles behave in a statistically measurable manner. However, when the drug is in the form of a heavier suspension, other strategies are required.
For example, a vaccine that appears as a resuspension in a syringe cannot be completely mixed. Although checking for particles adhering to the inner wall of the syringe, the noise associated with the rotation in the image makes it difficult to detect small particles in the syringe.
The liquid part is also difficult to distinguish transparent particles (for example, white fibers and glass). To solve this problem, continuous rotation is used for particle inspection. Each position in the turntable has a separate servo rotation unit with a specific rotation plan.
The positions of some particles are correlated and it is found that they are different relative to their position at the first station. From this change in position, it can be concluded that these are moving particles.
For highly viscous products such as gels or hyaluronic acid, the detection process cannot rely on rotating liquid to distinguish particle movement.
In this case, the container keeps rotating to cover the entire 360° façade and track all visible potential contaminants, as shown by the red trajectory in Figure 3 below.
The apparent velocity of potential contaminants is used to determine whether they are inside or outside the container. They move on “different radii” at the same angular rate, covering different displacements.
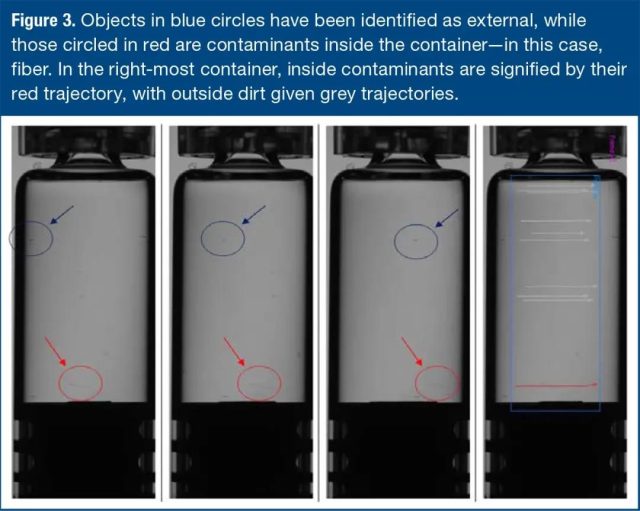 Figure 3. The object in the blue circle has been recognized as an external object, and the object in the red circle is the contaminant in the container-in this case, the fiber. In the rightmost container, internal contaminants are represented by red traces, and external fouling is represented by gray traces.
Figure 3. The object in the blue circle has been recognized as an external object, and the object in the red circle is the contaminant in the container-in this case, the fiber. In the rightmost container, internal contaminants are represented by red traces, and external fouling is represented by gray traces.
For these drugs, proper lighting schemes become particularly important because certain contaminants, such as white fibers, are easier to see against dark backgrounds. Similarly, it is easier to find many appearance defects, such as small scratches, on a dark background.
Biopharmaceutical drugs (such as monoclonal antibodies) may be more difficult to detect than other liquids because of their higher density, higher turbidity, and greater need to protect product integrity, which limits the available detection methods.
The trend of higher protein concentration and the resulting higher viscosity presents another challenge for detection, because the “spin and stop” detection technique becomes ineffective for particle detection with viscosities higher than 4-5 centipoise.
Turbidity also increases with increasing protein concentration, which makes distinguishing acceptable particles from unacceptable particles a real challenge. If the process-related impurities are within the scope of the pharmacopoeial guidelines for size and quantity, they are not necessarily harmful.
However, when the current automatic visual inspection system is used for inspection, due to the increased difficulty of accurately measuring and counting particles, a high rejection rate may occur. Therefore, manual inspection of biotechnology products has become the norm.
A more effective method is an automated detection system, which can identify, determine and count each particle in the container, and use historical particle data in clinical batches for patient safety-based assessments to determine whether it needs to be discarded.
By eliminating mechanical shock during processing, protein instability and clumping can be minimized. The main driving force of protein aggregation is the cavitation effect caused in the liquid, which increases the agitation of the gas-liquid interface and increases the local temperature and pressure of the liquid.
While considering the overall throughput, the inspection equipment must be able to handle the product smoothly.
The second driving factor of protein instability is the shear force exerted by high-speed rotation in the liquid, especially when acceleration and deceleration cause the transition from laminar to turbulent flow, which makes the meniscus unstable and creates bubbles in the liquid . In order to reduce the applied shear force, it is recommended to add a cross-correlation inspection system between the particle stations, so that sequence comparisons can be performed to determine whether the particles are inside or outside the syringe.
Using this method, the container is kept rotating at a steady speed, and the liquid is slowly accelerated to separate the bubbles at the top and bottom in a controlled manner, while the particles are located at different heights and can be detected during the inspection.
Conclusion
Syringe inspection is still one of the most challenging aspects of the post-pharmaceutical quality control process.
When the container and its ingredients must be thoroughly inspected, the syringe and the various drugs it provides have unique pain points in handling, visual inspection, and minimizing false alarms.
Best practice methods for syringe inspection means careful consideration of the type of syringe and the types of ingredients it contains, as well as the best use of lighting, rotation speed, and inspection techniques and methods.
Reference:
“Automatic Visual Inspection of Difficult-to-Inspect Lyophilized And Opaque Products on a Combi Machine,” Presentation at PDA Visual Inspection Forum (October 2020).
What’s syringe check and how to conduct syringe check?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



