FDA documents: Side effects of COVID-19 vaccine in children aged 5-11
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA documents: Side effects of COVID-19 vaccine in children aged 5-11
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA documents: Side effects of COVID-19 vaccine in children aged 5-11.
On October 22, 2021, the U.S. Food and Drug Administration (FDA) website published 82 pages of vaccine data for children aged 5-11 years that will be reviewed on the 26th of this month.
How serious is the children’s COVID-19 epidemic in the United States?
According to data from the American Academy of Children (AAP), as of October 14, 2021, among the 75 million children under the age of 18 in the United States, 6.17 million children with COVID-19 have been reported, accounting for 8.2% of all children!
Last week alone, more than 130,000 children were infected with COVID-19.
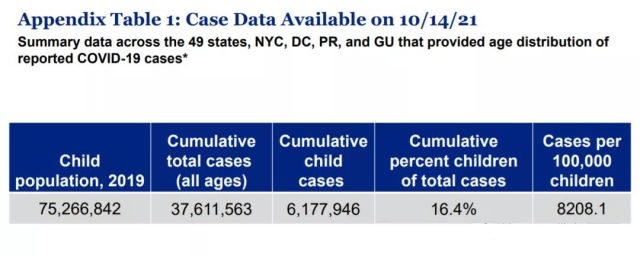 (Source: American AAP)
(Source: American AAP)
Under such circumstances, parents are looking forward to the long-standing childhood vaccine. Nevertheless, the supervision of vaccine data has not been relaxed in the slightest.
On October 22, 2021, the U.S. Food and Drug Administration (FDA) website published 82 pages of vaccine data for children aged 5-11 years that will be reviewed on the 26th of this month.
 (Source: US FDA)
(Source: US FDA)
Vaccine protection
The key data:
Pfizer-BioNTech’s BNT162b2 mRNA vaccine (children’s dose 10ug) has a significant effect in children aged 5-11, and its two doses have a protective effect of 90.7% in an environment where the Delta mutant strain is prevalent.
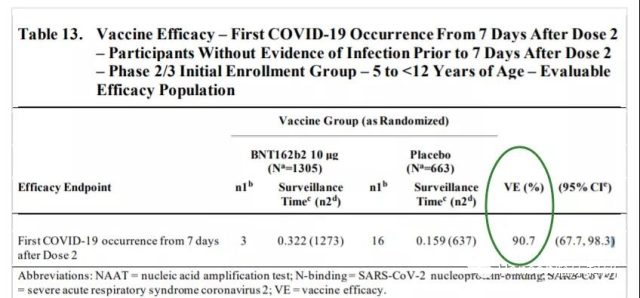 (Vaccine protection effect. Source: US FDA)
(Vaccine protection effect. Source: US FDA)
There is no need to worry about the protective power of BNT162b2, because on September 21, Pfizer has already announced the neutralizing antibody data.
One month after the second dose of the vaccination group, the induced geometric mean titer (GMT) of neutralizing antibodies was 1,197.6 (95% CI 1106.1-1296.6); it was equivalent to the GMT of 1146.5 for 30ug vaccinators in the 16-25 age group .
The results of clinical trials of mRNA vaccines for children aged 5-11 are finally here! Parents of elementary school students can’t wait for a long time.
Adverse reactions
What we are most concerned about is what are the adverse effects of the vaccine for children aged 5-11, so that we can prepare in advance.
Local reaction
The local adverse reactions of the vaccine are redness, swelling and pain at the injection site.
As shown in the figure below, we have marked the incidence of these three local adverse reactions at a dose of 10ug. After the second dose of vaccination, the incidence of redness, swelling, and pain were 37.5%, 31.3%, and 87.5%, respectively.
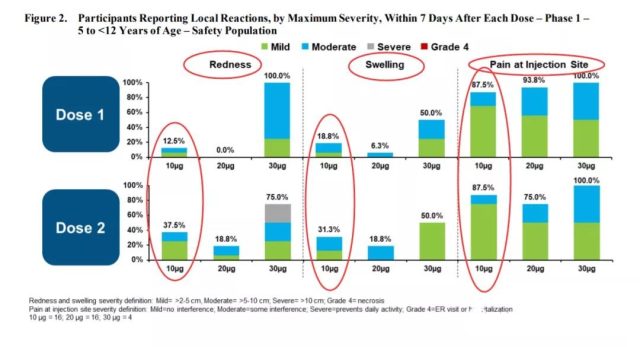 (Local vaccine reaction. Source: US FDA)
(Local vaccine reaction. Source: US FDA)
Systemic reaction
Including fever, fatigue, headache, chills, vomiting, diarrhea, muscle pain and joint pain.
It can be seen that after the second dose of vaccination, some children’s body temperature can reach 38.9-40°C, and half of the children have headaches.
Think of it after I vaccinated the second dose of vaccine (Moderna, 100ug), I took two painkillers Cai Guoguan. Therefore, under the guidance of a pediatrician, it is still necessary to prepare a little medicine for the child in advance.
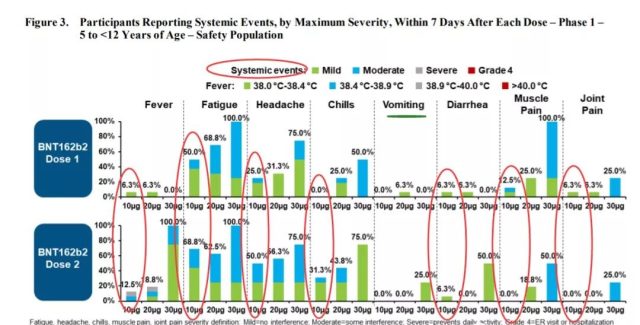 (Vaccine systemic reaction. Source: US FDA)
(Vaccine systemic reaction. Source: US FDA)
In another comparison between 5-11-year-old children and 16-25-year-old adolescents, it can be seen that after the second dose of vaccination, the 5-11-year-old age group even experienced serious adverse reactions with a body temperature of more than 40°C; however, The overall systemic adverse reactions are less than those in the 16-25 age group.
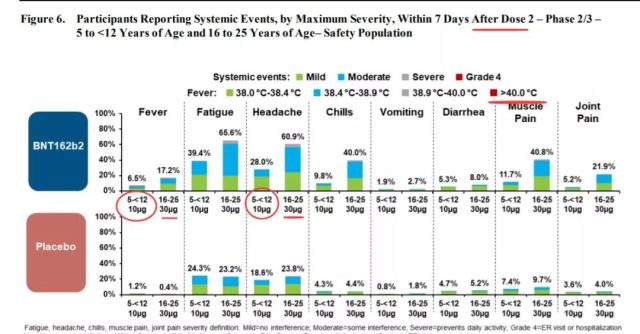 (Vaccine systemic reaction. Source: US FDA)
(Vaccine systemic reaction. Source: US FDA)
FDA documents: Side effects of COVID-19 vaccine in children aged 5-11
https://www.nejm.org/doi/pdf/10.1056/NEJMc2114290?articleTools=true
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



