Stem cells make patients to regenerate stable insulin after 90 days
- FDA Approved Opdualag: The First Immunotherapy Targeting LAG-3
- Cutaneous Reactions Following COVID-19 Vaccination: A Review of the Evidence
- Brief Intermittent Exercise Reduces Heart Disease and Death Risk
- Personalized Lung Tumor Chips Assess PD-1 Therapy Response
- Study Shows Prior Infection Offers Strong Immunity to Original COVID-19 Strain
- Chinese Food Products Dominate Korean Tables Amid Safety Concerns
Stem cells successfully make patients with type 1 diabetes to regenerate stable insulin after 90 days
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Stem cells make patients to regenerate stable insulin after 90 days.
Unprecedented! Stem cells successfully treat patients with type 1 diabetes and regenerate stable insulin after 90 days
Recently, Vertex Pharmaceuticals reported the first three-month positive data for patients with type 1 diabetes who participated in the VX-880 Phase I/II trial. VX-880 is the company’s experimental stem cell-derived islet cell replacement therapy.
Type 1 diabetes (T1D) is an autoimmune disease in which the host immune system mistakenly destroys the beta cells located in the islets of Langerhans. T1D was a fatal metabolic disease before the discovery of insulin in the early 20th century.
Recently, Vertex Pharmaceuticals reported the first three-month positive data for patients with type 1 diabetes who participated in the VX-880 Phase I/II trial .
VX-880 is the company’s experimentalStem cell- derived islet cell replacement therapy.
Type 1 diabetes (TlD) is an autoimmune disease, in which the host immune system mistakenly destroys the β Langerhans cells located in the islets. T1D was a fatal metabolic disease before insulin was discovered in the early 20th century .
People with T1D still need to take exogenous insulin every day to survive. At present, islet transplantation is the only source of exogenous β-cells.
Currently, more than 50% of pancreatic islet transplant recipients maintain insulin independence and/or restore hypoglycemic function for up to 5 years after transplantation.
VX-880 is a research allogeneicStem cell- derived, fully differentiated, insulin-producing islet cell therapy is manufactured using proprietary technology.
VX-880 may restore the body’s ability to regulate glucose levels by restoring pancreatic islet cell function, including the production of glucose in response to insulin.
VX-880 is delivered by injection into the hepatic portal vein and requires long-term immunosuppressive therapy to protect islet cells from immune rejection.
According to Vertex, the results showed that VX-880 restored insulin production in the patient who received a single infusion of half the target dose of VX-880 combined with immunosuppressive therapy.
Patients have also achieved “quick and steady” benefits on a number of measures, including improved blood sugar control (including HbA1c) and a reduction in daily insulin requirements.
The patient was diagnosed with T1D about 40 years ago and has been dependent on exogenous insulin. One year before treatment, the patient experienced 5 severe and potentially life-threatening episodes of hypoglycemia.
Before treatment with VX-880, the patient’s insulin dose was 34 units per day, and fasting and stimulated C-peptide levels could not be detected, indicating that the patient did not make insulin by themselves.
According to the study protocol, patients received half the target dose of VX-880 through the hepatic portal vein, combined with the standard immunosuppressant regimen.
Fasting C-peptide, HbA1c and 7 balances are daily insulin dose of VX-880 after treatment to day 90 at different time intervals measured. On the 90th day after VX-880 treatment, the patient’s fasting C-peptide was 280 pmol/L, reflecting the recovery of basal insulin production.
After MMTT stimulation, it increased to a peak of 560 pmol/L, indicating that VX-880 restored glucose-responsive insulin production. Also on Day 90, HbA1c increased from 8.6% at baseline to 7.2%, and the daily insulin dose decreased from 34 units per day before VX-880 treatment to an average of 2.9 units per day during the 7-day period (Table 1). On the 90th day, the patient’s daily exogenous insulin consumption was reduced by 91%.
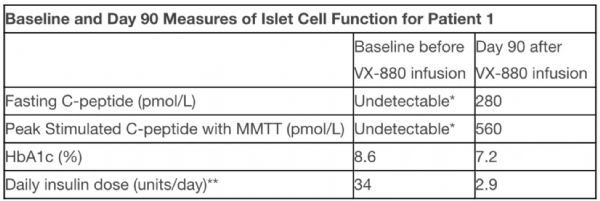
Table 1. Patients have also achieved “fast and robust” benefits in a number of measures
Bastiano Sanna, head of cell and gene therapy at Vertex, called these results “unprecedented.” What really sets them apart is that they were achieved at half the target dose. Although it is still too early, these results support the continued progress of VX-880 clinical research.
Original source:
https://www.firstwordpharma.com/node/1871904?tsid=28®ion_id=6
Stem cells successfully make patients with type 1 diabetes to regenerate stable insulin after 90 days
(sourceinternet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.