Will immunotherapy based on NKT cells shine in the future?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Will immunotherapy based on NKT cells shine in the future?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Will immunotherapy based on NKT cells shine in the future?
At present, tumor immunotherapy is in the ascendant, and one of the most effective treatment strategies is adoptive cell transfer therapy (ACT).
Chimeric antigen receptor (CAR) and engineered T cell receptor (TCR) are the main adoptive T cell immunotherapy in recent years .
Natural killer T (NKT) cells are a subset of lipid-responsive T cells that can enhance anti-tumor immunity.
The immunotherapy based on NKT cells has shown promising therapeutic effects in pre-clinical treatment, but has not achieved significant effects in clinical treatment.
At present, some new treatment strategies are being developed and applied to NKT cell therapy , which is expected to make breakthroughs in future clinical treatments.
NKT cells
NKT cells are a subset of lipid- and glycolipid-responsive T lymphocytes that co-express NK cell-related markers (NKp46, NK1.1).
NKT cells play an important role in tumor immune monitoring and anti-tumor immunity. Unlike traditional T cells that recognize peptide antigens by MHC I or II, NKT cells recognize endogenous and exogenous glycolipids presented by MHC I-like molecules CD1d.
Based on TCR rearrangement and glycolipid responsiveness, NKT cells can be divided into two main subgroups: type I and type II NKT cells.
Type I NKT (also known as iNKT) cells express constant rearrangement of TCRα chains and restricted TCRβ chains.
In mice, Vα14Jα18 (TRAV11-TRAJ18) is paired with Vβ7 (TRB29), Vβ8.2 (TRB13-2) or Vβ2 (TRBV1); in humans, Vα24Jα18 (TRAV10-TRAJ18) is paired with Vβ11 (TRBV25-1).
After recognizing glycolipid antigens, iNKT cells rapidly secrete immunomodulatory cytokines, including IFN-γ, TNF and IL-4, to affect downstream immune activity.
Human iNKT cells can be CD4, CD8 double negative (CD4-CD8-), while mouse NKT cells are CD4+ or CD4-CD8-. In addition, iNKT cells can differentiate into NKT1, NKT2 and NKT17 subpopulations, similar to Th1, Th2 and Th17 T cell subpopulations.
Type II NKT cells have more diverse Vα rearrangements (including TRAV7, TRAV9, and TRAV12), which help recognize their own lipids, such as cerebroside sulfate or lysophosphatidylcholine.
A number of studies have shown that activating type II NKT cells by administering cerebroside sulfate can increase tumor growth and enhance metastasis, indicating that it has a tumor-promoting effect, while iNKT cells show strong anti-tumor activity.
iNKT cell-mediated anti-tumor effect
Activated iNKT cells can provide anti-tumor immunity through four mechanisms: direct tumor lysis, recruitment and activation of cytotoxic innate and adaptive immune cells, suppression of suppressor cells in the tumor microenvironment (TME), and promotion of tumor-targeted immune memory.
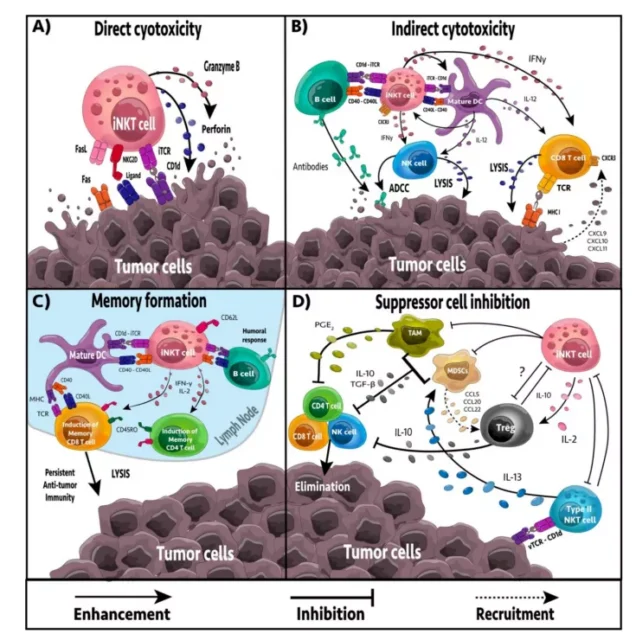
2.1 Direct or indirect targeting
iNKT cells can directly or indirectly target tumor cells. iNKT cells have the ability to mediate direct cytolytic activity against CD1d-positive tumor cells through perforin, granzyme B, and TNF-related apoptosis-inducing ligand (TRAIL) pathways.
In vitro and in vivo studies have shown that iNKT cell-mediated cytotoxicity is related to the increased expression of CD1d on the surface of tumor cells, leading to enhanced tumor cell lysis and decreased metastasis rate, while the down-regulation of CD1d is related to decreased iNKT cell recognition, tumor escape and increased malignancy.
In the absence of CD1d expression on tumor cells, iNKT cells may be activated by CD1d+APC, including dendritic cells (DC), B cells, myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAM).
The response of iNKT cells largely depends on the APC presenting glycolipids. After activation, iNKT cells can secrete a large number of pro-inflammatory cytokines, including IL-2, IL-4, IL-17, IFN-γ and TNF.
These cytokines affect a wide range of immune cells, including DC, macrophages, and neutral cells. Granulocytes, NK cells, and T and B cells.
In addition, activated iNKT cells can actively recruit and induce DC maturation through the interaction of CD40/CD40L and CD1d/TCR.
2.2 Regulation of immunosuppression
TAM is an immunosuppressive immune cell that frequently appears in TME. TAM contributes to the inhibition of tumor progression and NK, iNKT and T cell responses. In neuroblastoma, iNKT cells can co-localize and lyse TAM through a CD1d-dependent mechanism.
In addition, iNKT cells can reprogram M2 polarized TAM into inflammatory M1 macrophages, reducing TAM-mediated immune suppression.
MDSC induces anergy of NK and T cells, promotes immunosuppression and tumor growth. In addition, they can reshape TME and increase the transfer rate. In the influenza A infection model, iNKT cells can inhibit the inhibitory activity of MDSCs mediated by arginase-1 and NOS2 in a CD1d and CD40-dependent manner.
In addition, NKT cells can stimulate the conversion of α-GalCer-loaded MDSCs into mature APCs, which can induce NK and T cell immune responses.
In addition, type II NKT cells appear more frequently in TME, have immunosuppressive effects, and increase tumor progression and metastasis. The activation of iNKT cells reduces the number of type II NKT cells.
2.3 The formation of tumor immune memory
The generation of immune memory is essential for a durable anti-tumor immune response and the prevention of tumor recurrence.
The activation of tumor antigens or cross-reactive foreign antigens can induce the expansion and differentiation of naive T cells to obtain appropriate effector functions to target cancer cells.
CD8α+ dendritic cells perform well in MHC I cross-presentation. It has been proven that iNKT cells can directly promote CD8α+ dendritic cells to cross-prime, even in the absence of CD4+ T cells.
In a variety of tumor models, NKT cell activation has been shown to enhance CD8+T cell-mediated anti-tumor immunity.
Although most iNKT cells lack CD62L, a recent study showed that after glycolipid stimulation, CD62L+iNKT cells increase and last longer than CD62Lneg iNKT cells.
CD62Lneg iNKT cells acquire an exhausted phenotype (PD-1, TIM-3, reduced cytokine production) and rapidly undergo apoptosis, while CD62L+iNKT cells continue to proliferate and produce a large number of cytokines.
NKT-based cellular immunotherapy
Due to the important role of iNKT cells in immune monitoring and anti-tumor immunity, immunotherapy based on iNKT cells has become an important research field, and a variety of strategies have been used to target cancer.
3.1 Give free α-GalCer
In preclinical trials, free α-GalCer can be administered prophylactically, simultaneously or after tumor inoculation. After injection of free α-GalCer, iNKT cells proliferate rapidly and produce TNF, IL-2, IL-4 and IL-13, followed by IFN-γ response, and multiple injections are biased towards Th2 response.
Although in multiple mouse models, the administration of free α-GalCer after tumor inoculation has a certain anti-tumor effect, but the anti-tumor response is limited.
Therefore, free α-GalCer is usually used in combination with cytokines or other immunotherapies to improve the therapeutic effect. In the phase I clinical trial, the administration of α-GalCer was well tolerated and had no dose-limiting toxicity; however, it failed to produce a clinical response, with only 7 SD out of 24 patients.
The low efficacy may be attributed to low iNKT cell numbers at baseline, because only patients with high iNKT cell numbers showed NK cell toxicity and stable disease.
3.2 DCs presented by adoptive transfer of α-GalCer
DC loaded with α-GalCer has been used to overcome the low immunogenicity and incompetent induction of free α-GalCer. Due to the co-stimulatory signal transduction of CD40 and IL-12, loading α-GalCer on DC will lead to stronger activation of iNKT cells and less induction of incompetence.
In a variety of tumor models, α-GalCer-loaded DCs can reduce tumor growth and metastasis compared with the administration of free glycolipids, thereby prolonging survival. DC loaded with α-GalCer increased the proliferation and activation of iNKT and the production of IFN-γ. In addition, the enhanced activation of iNKT cells leads to the activation of NK and CD8+ T cells and increased IFN-γ production.
In clinical trials of myeloma and head and neck cancer, mature APC loaded with α-GalCer was well tolerated without serious adverse events.
The treatment increased the production of IFN-γ and the expansion of iNKT cells, resulting in many patients with stable disease and prolonged median survival time.
In the phase I and phase I/II clinical trials of non-small cell lung cancer, the treatment of APCs loaded with α-GalCer was well tolerated, resulting in only grade I and II adverse events.
Patients who responded well to treatment showed increased IFN-γ production and iNKT cell expansion. The median survival time was 31.9 months, while the median survival time for all patients was 18.6 months.
3.3 Adoptive transfer of activated iNKT cells
Cancer patients usually have a small number of iNKT cells and/or reduced function, which limits the efficacy of α-GalCer treatment. In order to overcome this limitation, iNKT cells have been isolated from patient-derived PBMC and expanded in vitro and then adoptively transferred back to the patient. In preclinical models, iNKT cells activated by adoptive transfer can increase the cytotoxicity, tumor regression and overall survival rate of iNKT cells in the melanoma metastasis model.
On the contrary, in the metastatic 4T1 breast cancer model, the adoption of NKT cells alone or in combination with free α-GalCer therapy or α-GalCer loaded DCs did not improve the prognosis.
Clinical trials of adoptive transfer of iNKT cells for the treatment of non-small lung cancer and advanced melanoma did not report serious adverse events.
The number of circulating iNKT cells and the production of IFN-γ increased in patients, but few patients had reduced tumor progression, indicating that the treatment was ineffective. Combining adoptive transfer of expanded iNKT cells with DC loaded with α-GalCer can improve the efficiency of adoptive transfer of NKT cells.
Compared with adoptive transfer of iNKT alone, the combination therapy can make some patients get a partial response.
3.4 As a cancer vaccine adjuvant
The role of iNKT cells in recognizing conserved lipid antigens presented by CD1d and their ability to coordinate anti-tumor immune responses through cytokine signals make them attractive targets for cancer vaccine development.
INKT cell ligands, such as α-GalCer, can be used together with tumor antigens to act as an adjuvant and increase the adaptive immune response to tumors.
In preclinical animal models, α-GalCer combined with tumor antigens can improve the overall survival rate of mice.
Intranasal vaccination with α-GalCer and OVA can induce humoral and cellular immune responses, leading to increased cytokine secretion, CTL response and IgG production. Currently, there are no registered clinical trials for the use of iNKT cell ligands in cancer vaccines.
3.5 CD1d antibody fusion protein
CD1d-positive tumors are more susceptible to iNKT cell-mediated lysis, and CD1d down-regulation is a common evasion strategy to avoid detection. Therefore, in order to increase the targeting of iNKT cells to tumors, a CD1d antibody fusion protein that directs iNKT cells to tumors was studied.
Currently, CD1d antibody fusion proteins targeting HER2, CEA and CD19 have been developed. In the B16 melanoma model expressing HER2, CD1d antibody fusion protein treatment targeting HER2 increased iNKT cell inflammatory cytokine production and targeted tumor cell lysis, and reduced metastasis formation. In addition, iNKT cells enhance the activation of DC, NK cells and CD8 T cells, increasing the overall immune recruitment and targeting of tumors. Importantly, the CD1d antibody fusion protein has target specificity and shows good safety.
3.6 CAR-NKT cells
CAR-T cell therapy has achieved significant clinical success in targeting B cell malignancies. iNKT cells have been designed to express CARs targeting glycolipids and protein antigens.
In addition, in addition to CAR, CAR-NKT cells also co-express a constant TCR to maintain their reactivity to glycolipid antigens. In contrast, the endogenous TCR in CAR-T cells is polyclonal, and most CAR-T cells can only respond through their CAR.
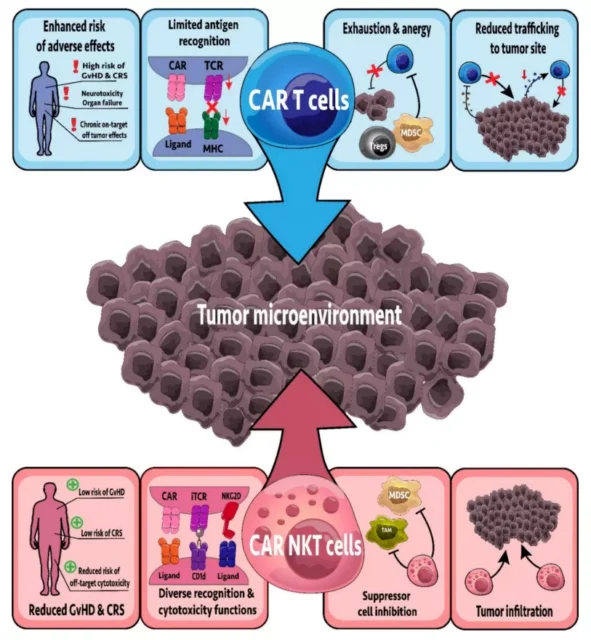
CAR-NKT cells targeting CSPG4, GD2 and CD19 have been developed, and CAR-NKT cells targeting GD2 and CD19 are undergoing clinical trials.
A phase I clinical trial (NCT03294954) has begun to detect the efficacy and safety of anti-GD2 CAR-NKT cells in refractory neuroblastoma.
Preliminary results showed that the treatment of 10 selected patients was safe, of which 1 had a complete remission, 1 had a partial remission, and 3 patients were in stable condition.
Currently, CD19 CAR-NKT cells are undergoing a phase I clinical trial (NCT03774654) to detect the safety and effectiveness of relapsed and refractory B-cell malignancies.
Of the two patients in the phase I trial, one patient showed a complete response and the other showed a partial response, and neither patient showed any signs of CRS or GvHD.
Importantly, the CAR-NKT cells used in this study are allogeneic, proving the potential of “universal” therapy.
Combination therapy based on NKT cells
One way to improve existing therapies is to combine them with other therapies that produce a synergistic response.
Combination therapy may also improve the clinical efficacy of iNKT cell immunotherapy. In preclinical experiments, iNKT cell immunotherapy has been tested in combination with chemotherapy, oncolytic viruses and other immunotherapies.
4.1 Combination chemotherapy
So far, lenalidomide is the only chemotherapy drug used in combination with iNKT cell immunotherapy in clinical trials.
In a phase I clinical trial, patients with asymptomatic myeloma received α-GalCer-loaded DC combined with lenalidomide.
The treatment was well tolerated, and only one patient had a grade 3 adverse event. The treatment resulted in increased activation of iNKT cells, NK cells, monocytes and eosinophils, showing strong innate immune activation.
In addition, the expression of NKG2D on NK cells was significantly increased, indicating that the cytotoxic potential of NK cells was increased.
In general, the treatment resulted in a decrease in tumor-associated immunoglobulins in all patients except one patient, indicating that the treatment reduced the tumor burden.
The success of the combination therapy emphasizes the need for further clinical trials.
4.2 Combined with oncolytic virus
To date, no clinical trials have combined oncolytic viruses with iNKT cell immunotherapy.
However, clinical trials have shown that oncolytic viruses can improve the effects of other immunotherapies.
The successful preclinical results of the combination of oncolytic virus therapy and iNKT cell activation therapy indicate that it is necessary to further examine this combination therapy in clinical trials.
4.3 Combined immunotherapy
An interesting option for combination therapy is the combination of iNKT cell therapy and immune checkpoint inhibitors.
After α-GalCer activates iNKT cells, IFN-γ is released in large quantities, and then the expression of PD-1 increases, leading to impotence and inhibition of anti-tumor function.
Therefore, the combination of iNKT cell immunotherapy and checkpoint inhibitors that block the PD-1/PD-L1 axis may improve the therapeutic effect.
In fact, in preclinical models, α-GalCer combined with anti-PD-1 or anti-PD-L1 can prevent the inability of iNKT cells to increase the anti-tumor activity of iNKT cells.
In addition, iNKT cell immunotherapy can overcome the incompetence of CD8+ T cells in PD-1 resistant tumors. Clinical trials are testing the combination of PD-1/PDL-1 blockers and iNKT cell immunotherapy (NCT03897543).
Challenges and countermeasures of NKT cell therapy
The biggest challenge facing iNKT cell immunotherapy is the low infiltration and reduced function of iNKT cells in cancer patients.
Coupled with the down-regulation of tumor cells CD1d, the therapeutic effect may be limited. In addition, iNKT cells induced an anergy phenotype after treatment with free α-GalCer, which reduced the efficacy of multi-dose treatment.
Another challenge is to obtain a large number of autologous DC and iNKT cells from immunosuppressed cancer patients, thereby limiting the number of cells that can be expanded and adopted.
In addition, it takes several weeks for culturing and differentiated cells to undergo adoptive transfer, resulting in some patients being unable to receive treatment or dying of disease.
In order to meet these challenges, many studies have improved iNKT cell immunotherapy.
5.1 Improved glycolipid delivery
Due to the inefficiency of free α-GalCer and the difficulty of obtaining a large number of autologous DCs, some studies have tested the possibility of α-GalCer delivery via carriers (such as nanoparticles, artificial antigen presenting cells, exosomes, and liposomes).
Compared with free α-GalCer, delivery of carrier-bound α-GalCer increases iNKT cell expansion and cytokine release, thereby reducing tumor burden.
This is mainly due to the increased uptake and expression of DC cells, as well as the increase in downstream NK and CD8+ T cell responses.
5.2 Choosing a glycolipid substitute
Almost all clinical trials of iNKT cell immunotherapy use α-GalCer (KRN7000) to stimulate iNKT cells; however, more and more modified glycolipids may provide higher therapeutic benefits.
For example, α-C-GalCer replaces the O-glycosidic bonds found in α-GalCer with C-glycosidic bonds, thereby increasing the production of IFN-γ and IL-12.
7DW8-5 is a candidate glycolipid with a short acyl chain and a fluorinated benzene ring at the end. Compared with α-GalCer, 7DW8-5 has a stronger affinity for the CD1d and TCR of iNKT cells.
In a variety of vaccine models, 7DW8-5 stimulates iNKT cells to increase Th1 and CD8+T cell responses.
Similarly, a modified glycolipid called ABX196 has produced promising results as a vaccine adjuvant. In melanoma, colon cancer, and bladder cancer models, ABX196 alone or in combination with anti-PD-1 can increase tumor regression and overall survival.
ABX196 combined with PD-1 inhibitor is currently undergoing a phase I clinical trial (NCT03897543).
5.3 iNKT cells derived from induced pluripotent stem cells
Cancer patients usually reduce the number and function of iNKT cells, which limits the efficacy of iNKT cell immunotherapy.
In addition, it is difficult to obtain enough PBMC from immunosuppressed cancer patients to expand and deliver iNKT cells in vitro. One coping strategy is to use induced pluripotent stem cells (IPSC) from patient tissues.
iPSC-induced iNKT cells can secrete large amounts of IFN-γ. In addition, in vivo experiments have shown that iPSC-derived iNKT cells retain their anti-tumor function when transferred to mice.
5.4 Targeting type II NKT cells
iNKT cells and type II NKT cells play opposite roles in tumor immune regulation and cross-regulate each other.
Therefore, targeting type II NKT cells may be a therapeutic method to alleviate tumor-mediated iNKT cell immunosuppression and enhance anti-tumor immunity. C24:2, a subtype of cerebroside sulfate antigen of type II NKT cells recently discovered, has been shown to significantly reduce the occurrence of lung metastasis.
Summary
iNKT cells play an important role in immune monitoring and anti-tumor immunity. NKT cell-based immunotherapy has many advantages.
Compared with CAR-T cell therapy, NKT cell therapy lasts longer and can inhibit the recurrence and metastasis of cancer. It may become an important treatment for solid tumors and other cancers.
Although there is still no breakthrough in clinical research, some new treatment strategies, including carrier-based α-GalCer, alternative glycolipids, and combination therapies, are showing improved preclinical results.
With the continuous breakthrough of immune cell therapy technology for solid tumors, the NKT cell immunotherapy industry will develop rapidly, and it is believed that immunotherapy based on NKT cells will shine in the future.
references:
1.The Current Landscape of NKT CellImmunotherapy and the Hills Ahead. Cancers (Basel). 2021 Oct; 13(20): 5174.
Will immunotherapy based on NKT cells shine in the future?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



