Immunotherapy combined with Radiation Chemotherapy Targeted therapy…
- FDA Approved Opdualag: The First Immunotherapy Targeting LAG-3
- Cutaneous Reactions Following COVID-19 Vaccination: A Review of the Evidence
- Brief Intermittent Exercise Reduces Heart Disease and Death Risk
- Personalized Lung Tumor Chips Assess PD-1 Therapy Response
- Study Shows Prior Infection Offers Strong Immunity to Original COVID-19 Strain
- Chinese Food Products Dominate Korean Tables Amid Safety Concerns
Immunotherapy combined with Radiation Chemotherapy Targeted therapy…
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Immunotherapy combined with radiation, chemotherapy, targeted therapy…
Immunotherapies such as immune checkpoint inhibitors ( ICIs ) and adoptive cell therapy ( ACT ) have revolutionized cancer treatment, especially metastatic cancers, where some patients who were previously considered incurable can achieve long-term remission and survival.
However, while immunotherapy can produce durable responses, ICI monotherapy typically has a response rate of only around 20% in solid tumors, and many patients eventually develop secondary resistance.
Once immune cells enter the tumor immune microenvironment ( TME ), many mechanisms arise to induce resistance against tumor immunity, including cancer cell-intrinsic factors, immunosuppressive cells, and the immunosuppressive environment.
Currently, one strategy to improve tumor immunotherapy is to develop biomarkers, such as PD-L1, that can be used to select potential responders or exclude potential non-responders.
Another strategy is to combine drugs with different mechanisms of action so that multiple resistance mechanisms can be overcome.
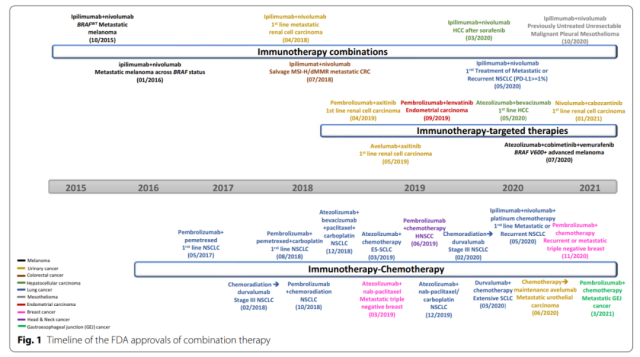
So far, the FDA has approved several combination therapies for different cancer types.
More combinations of immunotherapy and radiation, chemotherapy, targeted therapy, and immunotherapy are being tested clinically to target multiple defects in the immune cycle and intrinsic changes in cancer, thereby improving anticancer efficacy.
Immunotherapy combined with chemotherapy
Most chemotherapeutic drugs work through their direct cytotoxic effects, without considering the effects on the immune system.
However, the interaction between chemotherapy and immunotherapy has been demonstrated in mouse models, with mice with intact immune systems showing significantly improved tumor responses to anthracyclines.
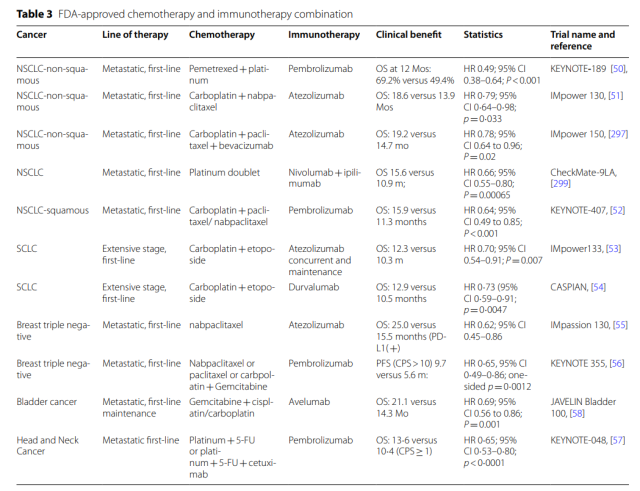
To date, multiple studies have demonstrated the contribution of cytotoxic chemotherapy to anticancer immunity, and there are several FDA-approved combination therapies with immunotherapy.
Chemotherapy drugs act synergistically in immunotherapy through multiple mechanisms:
Immunogenic Cell Death (ICD)
ICD is a regulated form of cell death that activates adaptive immune responses in immunocompetent hosts.
Numerous studies have shown that cytotoxic chemotherapy induces ICD and enhances immunotherapy.
Damage to cancer cells by cytotoxic chemotherapy results in release and relocation of damage-associated molecular patterns ( DAMPs );
release of intracellular molecules such as ATP that enhances APC recruitment;
proteins released by cancer cells such as HSP70, HSP90, and calcium Reticulin, which promotes phagocytosis of stressed cancer cells by dendritic cells;
cytoplasmic DNA and RNA stimulate the production of type I interferons and other pro-inflammatory cytokines via the cGAS/STING pathway, toll-like receptor 3 ( TLR3 ), and TLR9 secretion, etc.
Enhanced antigenicity of cancer cells
Many commonly used cytotoxic drugs, such as anthracyclines, cyclophosphamide, platinum, and taxanes, can target cell cycle progression in proliferating cells and induce apoptosis.
After tumor cell death, antigen-presenting cells engulf the dead tumor cells and present tumor neoantigens to immune cells.
In addition, several other studies have shown that cytotoxic drugs upregulate antigen presentation mechanisms.
For example, gemcitabine can significantly upregulate the expression of HLA-A, B and C by increasing the expression of β2-microglobulin and alter the pool of peptide antigens expressed on HLA class I.
Depletion of immunosuppressive cells
Several subsets of immune cells are known to suppress anticancer immunity.
Cytotoxic chemotherapy, such as platinum, cyclophosphamide, gemcitabine, and 5-fluorouracil, can significantly reduce MDSCs in humans and mice.
Trabectedin selectively depletes monocytes/macrophages by activating caspase-8-dependent apoptosis.
Human Treg cells lack expression of the cyclophosphamide metabolic transporter ABCB1 and are more sensitive to cyclophosphamide treatment than other immune cells.
Regulation of gene expression
Besides cytotoxic chemotherapy, another major class of small molecule drugs are epigenetic modulators.
Epigenetic regulation, such as DNA methylation, histone modifications, and chromatin remodeling, has a huge impact on tumorigenesis.
Besides direct induction of ICD and stimulation of antitumor immunity, another major mechanism of synergy between epigenetic modulators and immunotherapy is through gene expression modification.
Both HDAC and DNA methyltransferase ( DNMT ) inhibitors have been shown to upregulate antigen processing and presentation mechanisms.
They also upregulate costimulatory molecules such as CD80, CD86 and ICAM-1, as well as the immune checkpoints CTLA4, PD-1 and PD-L1.
In addition, cytokines can also be induced, and epigenetic modulators can enhance the response to immunotherapy.
Enhance and restore sensitivity to chemotherapy
Several studies have shown that boosting immunotherapy and cytotoxic chemotherapy are reciprocal.
Some patients with chemotherapy-resistant tumors respond to chemotherapy rechallenge after anti-PD-1 therapy.
In Hodgkin lymphoma and non-small cell lung cancer, increased responses to salvage chemotherapy were observed following disease progression with immune checkpoint blockade.
Adverse effects of chemotherapy on immunotherapy
Chemotherapy also has a detrimental effect on immunotherapy, and one of the major deleterious effects of chemotherapy on the immune system is lymphatic depletion, leading to immunosuppression.
In fact, some of the immunosuppressive drugs clinically used to treat autoimmune diseases are also used in cancer chemotherapy, but with different doses and timings.
Whether chemotherapy-induced lymphatic depletion has an inhibitory effect on anticancer immunity is still controversial.
Chemotherapy can also affect tertiary lymphoid structures ( TLS ).
TLSs are ectopic lymphoid tissues that form in non-lymphoid tissues, including cancer, that resemble secondary lymphoid organs such as lymph nodes .
Numerous data suggest that TLS functions like lymph nodes, recruiting lymphocytes to tumors and enhancing local and systemic immune responses to cancer.
Immunotherapy combined with radiotherapy
Stimulation of antitumor cancer immunity by radiotherapy ( RT ) has been proposed for the first time in a case report of distant untreated tumor regression after local radiotherapy .
Although this RT-induced distal effect is very rare and elusive, its role in inducing antitumor immune responses is intriguing and has been of great interest with the advent of immune checkpoint blockade.
The mechanism of action of radiotherapy to enhance immunotherapy
In addition to altering the local TME, RT can also enhance tumor antigenicity and adjuvant properties.
RT increases tumor antigenicity through multiple pathways.
First, similar to chemotherapy, radiation can induce MHC-I expression and enhance tumor antigen presentation. Second, radiation induces ICD.
During ICD, annexin A1 increases the interaction of antigen-presenting cells with dying cancer cells, while HSP70, HSP90, HMGB1, and other molecules promote T-cell uptake and cancer antigen presentation.
Third, radiation reduces CD47 expression on the cell surface, enhancing uptake and antigen presentation by cancer cells.
Fourth, reactive oxygen species ( ROS ) generated by ionizing radiation can modify macromolecules such as proteins and DNA, and increase antigenicity.
In addition to direct DNA damage, reactive oxygen species production is also critical for radiation-induced tissue damage.
Another important contribution of radiation to antitumor immunity is the enhancement of adjuvant effects.
Radiation-induced DNA damage and micronucleus DNA activate innate and adaptive immune responses through the cGAS/STING pathway and upregulate the expression of the type I interferon pathway.
In addition to the cGAS-STING pathway, release of ICDs, DAMPs, and cytokines can enhance adjuvant properties, induce migration of pro-anticancer immune subsets, reduce immunosuppressive cells, and enhance responses to cancer cell killing.
Adverse effects of radiotherapy on immunotherapy
On the other hand, there is also good evidence that radiation causes immunosuppressive TME. In addition to cancer cells, radiation can also kill normal cells, including immune cells.
Furthermore, radiation can alter the TME and induce an immunosuppressive milieu, and several studies have shown that radiation induces infiltration and aggregation of MDSCs and Tregs, reduces infiltration of CD8+ T cells, and promotes immunosuppressive TME through multiple pathways.
In addition, other mechanisms of radiation immunosuppressive effects include dysregulation of tumor blood vessels, hypoxia, stroma, tumor-associated macrophages ( TAMs ), cancer-associated fibroblasts ( CAFs ), cytokines, and more.
Clinical progress of radiotherapy combined with immunotherapy
The first report showing benefit of radiation therapy and immunotherapy came from a patient with melanoma who progressed while undergoing a clinical trial with ipilimumab, but then had tumor shrinkage following radiation therapy.
The KEYNOTE-001 trial showed that prior radiotherapy was associated with significant improvements in PFS and OS in NSCLC patients treated with pembrolizumab.
Since then, clinical trials of radiation therapy and immunotherapy have sprung up. Currently, there are more than 800 ongoing clinical trials.
To date, several clinical studies have shown improved clinical outcomes when radiation is added to ICIs.
According to the phase III PACIFIC trial, durvalumab has been approved as maintenance therapy after platinum-based chemoradiotherapy in patients with stage III NSCLC. Combining durvalumab significantly increased median PFS ( 17.2 months vs 5.6 months; p<0.001 ) and OS ( p=0.0025 ).
In addition to anti-PD1/PD-L1 antibodies, radiation therapy has been studied in combination with other immunotherapeutic agents such as cytokines, cell therapy, vaccines, and other immune checkpoint inhibitors .
While most studies are still ongoing, some early reports suggest that these combinations are feasible and may achieve synergistic effects.
Immunotherapy combined with targeted therapy
All cancers have genomic alterations that contribute to the development of cancer.
Targeting these genomic alterations can have direct antitumor activity and induce stronger responses than cytotoxic chemotherapeutics.
For example, in patients with non-small cell lung cancer, platinum-based response rates were less than 30%, but an 80% response rate was observed in patients with epidermal growth factor receptor ( EGFR ) driver mutations treated with erlotinib .
In addition, many molecular drivers affect multiple links in the cancer immune cycle. At present, the FDA has approved 6 combination therapy of targeted drugs and ICIs.
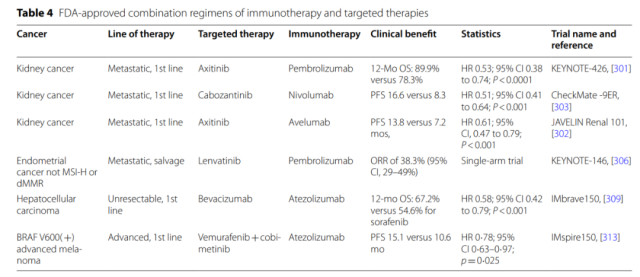
Targeted therapy enhances the mechanism of action of immunotherapy
Targeted drugs can eliminate cancer cells through direct antitumor activity and ICD, which can not only reduce the number of cells targeted and destroyed by immune cells, but also eliminate immunosuppressive factors and improve the effect of immunotherapy.
Furthermore, many oncogenic pathways are directly involved in regulating the expression of antigen presentation machinery.
The cyclin-dependent kinase 4 and 6 ( CDK4/6 ) pathway is commonly activated in many cancers, and inhibition of the CDK4/6 pathway can upregulate the expression of MHC. Similar findings were also found in the PI3K pathway.
Many abnormal signaling activities also have profound effects on immune cells. The VEGF-VEGFR pathway plays a key role in almost all immune cell subsets.
VEGFR is expressed on activated and memory T cells, and VEGF-VEGFR participates in the activation of downstream signaling pathways of T cells, inhibits TCR-dependent activation of T cells, and inhibits the cytotoxic activity of T cells.
In addition to Treg cells, VEGF can activate JAK2 and STAT3 and induce the accumulation of Gr1+CD11b+MDSCs.
In addition to direct antitumor activity, many signaling pathways have multiple functions in the TME and can influence antitumor immunity.
Activating mutations in EGFR occur in approximately 10–15% of non-small cell lung cancers and can upregulate PD-1 and PD-L1 expression, thereby mediating immune escape.
Similarly, activation of the PI3K/AKT pathway, including PTEN deletion, resulted in constitutive expression of PD-L1 and resistance to immunotherapy.
Indoleamine-2,3-dioxygenase ( IDO ) catalyzes the first and rate-limiting step of tryptophan catabolism, while the consumption of tryptophan and the accumulation of metabolites such as kynurenine Produces a high degree of immunosuppression and tolerance.
They can suppress effector T cell and NK cell function, stimulate Treg cells, promote the expansion of MDSCs, and polarize macrophages to an immunosuppressive M2 phenotype.
Clinical progress of targeted therapy combined with immunotherapy
Since many targeted therapy drugs can directly or indirectly modulate immune cell function, numerous clinical trials are currently underway to determine the effects of combined targeted therapy and immunotherapy.
Of these, antiangiogenic drugs may have the broadest immunomodulatory functions affecting nearly all immune cell subsets, so it is not surprising that five of the six FDA-approved targeted and immunotherapeutic combinations target angiogenesis.
The only targeted therapy combination that does not directly target angiogenesis is the BRAF inhibitor vemurafenib and the MEK inhibitor cobinetinib combined with atezolizumab in advanced melanoma with BRAF V600 activating mutations.
And three of the six combinations worked for advanced kidney cancer.
With the exception of anti-angiogenesis, nearly all targeted therapies that have been shown to modulate immune responses are currently being tested in combination with immunotherapies ( mainly ICIs ).
For example, more than 10 clinical trials are currently underway combining immunotherapy with drugs targeting the PI3K/AKT/mTOR pathway.
Furthermore, in addition to ICIs, targeted therapy is also being combined with other immunotherapy drugs.
For example, there are currently seven clinical trials combining BTK/ITK inhibitors with CAR-T cell therapy, and one clinical trial combining ibrutinib with a personalized peptide cancer vaccine.
Combination therapy with adoptive cell therapy
Adoptive cell therapy for cancer is the transfer of autologous or allogeneic immune cells into cancer patients to generate an anticancer immune response.
The first ACT was performed on patients with metastatic melanoma using autologous tumor-infiltrating lymphocytes ( TILs ).
With the development of chimeric antigen receptor ( CAR ) technology, the research and clinical application of CAR-T cell technology has been accelerated and achieved great success.
At present, the FDA has approved a number of CAR-T cell therapies. In addition to CAR-T, CAR-NK is currently a research hotspot, and a number of CAR-NK cell clinical trials are underway.
ACT and ICI
Following CAR-T cell infusion, PD-1 is upregulated, which can downregulate CD28 costimulatory signaling and induce CAR-T cell dysfunction.
Both preclinical and several clinical trials have shown that PD1/PD-L1 blockade and CAR-T combination therapy can achieve synergistic antitumor activity.
In order to eliminate the negative impact of the PD-1/PD-L1 axis on the function of CAR-T cells, CAR-T cells can be modified by knocking out the PD1-encoding gene PDCD1.
In addition to PD1/PD-L1, blockade of other immunosuppressive pathways has also been explored, for example, TGF-β is a major immunosuppressive regulator affecting multiple immune cells in the TME.
Knockdown of TGF-β signaling in CAR-T cells enhanced CAR-T cell proliferation and enhanced antitumor activity.
ACT combined with lymphatic clearance
Following CAR-T therapy, the recurrence of malignancies bearing the target antigen represents a potential opportunity for re-treatment with the same CAR-T cells.
However, in many cases, rechallenge with the same CAR-T cells often failed to induce a response.
One mechanism of resistance is that patients develop an immune response to the CAR.
To prevent immune responses to CARs, intensive lymphocyte depletion prior to CAR-T therapy has been used to improve clinical activity.
Furthermore, lymphoid clearance can minimize the influence of regulatory T cells, reduce other immune cells competing for homeostatic cytokines, and enhance APC activation.
Combination of CAR-T cell therapy
An alternative strategy to CAR-T combination therapy is to combine two types of CAR-T cells with CARs of different structures targeting the same target molecule, rather than using lymphoid clearance to block immune responses that eliminate CAR-T cells.
In this case, the second type of CAR-T cells survived and killed tumor cells even after the recipient patient had developed an immune response to the first type of CAR-T cells.
Another ACT combination strategy uses CAR-T cells that target two different antigens on tumor cells. One mechanism by which ACT fails is tumor cell heterogeneity, some of which do not express target molecules.
Targeting two different molecules to the same tumor maximizes tumor cell killing and reduces recurrence. This can be achieved by the tandem construction of a single CAR vector, or a combination of two different types of CAR-T cells, each targeting a different antigen.
ACT combined with immunomodulators
To create an immunostimulatory environment, CAR-T cells can be combined with other immunostimulatory molecules, such as immunostimulatory cytokines, cytokine receptors, and costimulatory molecules, and can even be modified to directly express these molecules, such as fourth-generation CAR-T cells.
IL-12 is one of the widely studied cytokines in preclinical models. It enhances the cytotoxic activity of CD8+ T cells and NK cells, stimulates Th1 helper T cell responses, and counteracts the immunosuppression of Treg and MDSCs. A fourth clinical trial of IL-12-expressing CAR-T cells has been initiated.
Combination Small Molecule Inhibitor
In addition to direct antitumor activity, the BTK inhibitor ibrutinib has several other immunomodulatory effects.
It downregulates PD-1 expression on CD4+ and CD8+ T cells, affects PD-L1 expression and IL-10 production on B cells in CLL.
In addition, ibrutinib preferentially inhibits Th2, favors Th1 differentiation, and converts MDSCs into dendritic cells.
Currently, several clinical trials of ibrutinib combined with CAR-T cell therapy are underway.
In addition to ibrutinib, several other tyrosine kinase inhibitors, such as EGFR inhibitors, are also being tested in combination with CAR-T.
Combined oncolytic virus
Currently, several preclinical studies have reported on the antitumor activity of ACT combined with oncolytic virus therapy.
Through this binding, oncolytic viruses can target and kill cancer cells, stimulate innate immune responses, and create a stimulatory immune environment to enhance ACT.
Because OVs are often tumor-targeted, they can express transgenes on the surface of cancer cells, which are then recognized by CAR-T cells.
Furthermore, armed OVs can express cytokines that attract CAR-T cells to the tumor site and enhance CAR-T cell cytotoxicity.
Combination therapy with cytokines
Cytokines interact with cell surface receptors and play key roles in regulating humoral and cellular immune responses by affecting cellular trafficking, maturation, growth, and reactivity of target cells.
Cytokines include chemokines, interleukins, interferons, and tumor necrosis factor. IL-2 and IFN-α are two cytokines approved for the treatment of cancer.
Because cytokines can be involved in regulating every process of the anticancer immune cycle, many clinical trials are underway combining cytokines with other drugs in the immune cycle to determine whether anticancer efficacy can be further improved.
For immunostimulatory cytokines, such as IL-2, IL-10, IL-12, and IL-15, natural forms and engineered cytokines have been combined with ICIs, e.g., pegylated long-acting IL-10 and anti-inflammatory cytokines A combination of PD1 antibodies, pembrolizumab or nivolumab, showed a manageable toxicity profile and preliminary antitumor activity.
For immunosuppressive cytokines, such as TGF-β, CCL2, and IL-8, neutralizing antibodies or small-molecule inhibitors have been tested clinically in combination with ICIs and chemotherapy.
Combination therapy for oncolytic viruses
Oncolytic virotherapy utilizes viruses to target and kill cancer cells, inducing innate and adaptive immune responses for cancer treatment.
This oncolytic activity enhances therapeutic advantage and induces immunogenicity after tumor cell death, resulting in increased infiltration of CD8+ T cells into the tumor microenvironment.
This important feature of oncolytic viruses can warm up immune “cold” tumors, which presents an enticing prospect in combination with other immunotherapies.
OV combined with chemotherapy
Temozolomide ( Temozolomide, TMZ ), an alkylating agent, is considered an effective anticancer drug for the treatment of various solid tumors, including gliomas and melanomas .
TMZ showed better antitumor effect in killing glioblastoma, lung cancer, melanoma and breast cancer by oncolytic herpes simplex virus, adenovirus, Newcastle disease virus and myxoma virus.
In a glioblastoma stem cell ( GSC ) model, the combined effect of oHSV G47Δ and TMZ resulted in robust DNA damage and prolonged survival in mice with GSC-derived intracranial tumors, achieving long-term remission in 50% of mice .
OV combined with targeted therapy
Oncolytic viruses interact with cancer-specific genes, proteins, or tissue environments during infection, replication, and release from cancer cells, potentially promoting tumor growth and survival.
This makes it possible for OVs to work synergistically with targeted therapy, which blocks tumor growth by interfering with specific molecules required for cancer and tumor growth.
Sorafenib, a targeted anticancer drug, is a tyrosine kinase inhibitor that inhibits multiple protein kinases, including VEGFR, PDGFR, and RAF kinases.
Heo et al. demonstrated the preclinical and clinical efficacy of the sequential combination of oncolytic poxvirus JX-594 and sorafenib in hepatocellular carcinoma ( HCC ).
OV and ICI
Preclinical studies have demonstrated that OV mJX-594 can sensitize ICI-resistant tumors and promote T-cell infiltration in mouse tumors, and in combination with anti-PD-1 therapy, reduces tumor growth by 70%.
Similarly, another study demonstrated that combined treatment with NDV and anti-CTLA-4 doubled protection from tumor recurrence and enhanced tumor lymphocyte infiltration compared to mice treated with anti-CTLA-4 alone.
Similar results were also confirmed in human trials. During clinical trials for the treatment of stage IIB-IV melanoma, the immune response of patients receiving T-VEC and treatment with ipilimumab was studied, and the combination therapy demonstrated increased CD4+ICOS+ compared to the limited response observed with ipilimumab monotherapy T cells are associated with significantly improved treatment outcomes.
The potential synergy between OVs and ICIs has made their combination in clinical trials popular, and multiple combinations are currently being evaluated.
OV combined with bispecific antibody
Bispecific antibody drugs have achieved preclinical and clinical success and are currently one of the hottest areas of research.
Nonetheless, bispecific antibodies are still limited by toxicity, half-life, tumor site retention capacity, and inability to generate durable immune memory.
To address this situation, an oncolytic adenovirus ( ICOVIR-15K ) was developed that was engineered to express an EGFR-targeting BiTE ( cBITE ). In co-culture assays, oncolysis resulted in T cell activation, proliferation, and enhanced cytotoxicity.
ICO15K-cBITE was shown to be tumor-selective, with intratumoral injection increasing the persistence and accumulation of tumor-infiltrating T cells in vivo compared to the parental virus, demonstrating enhanced antitumor activity in animal models.
Another example is an oncolytic virus expressing a fibroblast-activating protein ( FAP )-targeted BiTE ( fBiTE ).
In this way, immune cells are redirected to tumor stromal fibroblasts to improve tumor permeability and facilitate viral spread.
Furthermore, oncolytic viruses can be easily engineered into combinations of different immunotherapies, including BiTEs, cytokines, and ICIs.
CAdTrio, an adenovirus encoding IL-12, anti-PD-L1 antibody, and a specific BiTE against CD44v6, in combination with HER-2-CAR-T cells, significantly improved tumor control in a mouse animal model and survival rate.
Combination therapy with cancer vaccines
Cancer vaccines are designed to stimulate the first three steps of the anticancer immune cycle: cancer antigen release and presentation, immune cell priming, and immune cell activation.
Once immune cells are activated, they still need to complete the remaining steps: peripheral mobilization, penetration into the cancer site, recognition of cancer cells, and initiation of cytotoxicity against cancer cells.
Therefore, the resistance mechanisms of anticancer immunity, especially in the TME, still reduce the efficiency of cancer vaccines, and combination therapies to enhance cancer vaccines are currently being explored.
Several trials are currently underway to test the combined effects of cancer vaccines with cytokines or immune agonists.
For example, the combination of cancer vaccines with TLR-3 agonist multimeric ICLC to stimulate immune responses.
The immune response to the cancer/testis antigen NY-ESO-1 was higher when the NY-ESO-1 vaccine was combined with multimeric ICLC.
Furthermore, IL-2 plays a key role in the function of the immune response.
Compared with IL-2 alone, the combination of IL-2 with the tumor-associated antigen gp100 significantly improved ORR ( 16% vs 6%, p=0.03 ) and PFS ( 2.2 vs 1.6 months, p=0.008 ).
In addition to IL-2, several other immune-stimulating cytokines are under investigation, such as IL-12 currently undergoing several clinical trials to determine its efficacy and toxicity in combination with cancer vaccines.
The combination of cancer vaccines with ICIs is currently undergoing extensive preclinical studies and multiple clinical trials.
GX-188E is a therapeutic HPV DNA vaccine encoding HPV-16 and HPV-18 E6 and E7.
When GX-188E was combined with pembrolizumab in HPV-16/18-positive advanced cervical cancer, 11 of 26 patients achieved a response, and 4 patients ( 15% ) experienced a CR at 24 weeks.
This combination was well tolerated. Similar results were observed in another phase II trial of the synthetic long-peptide HPV-16 vaccine ISA101 in combination with nivolumab in HPV-16-positive solid tumors; the combination showed an ORR of 33% and a median 17.5-month bit OS.
summary
Due to tumor heterogeneity and complex immunosuppressive tumor microenvironment, single-agent immunotherapy often fails to overcome these factors, resulting in low response rates or secondary resistance.
Therefore, the direction of immunotherapy tends to be the combined application between different treatment methods.
Currently, the FDA has approved several combination therapies to improve the clinical efficacy of immunotherapy.
With the continuous deepening of reliable biomarkers and immuno-oncology mechanism research, more IO combinations including ACT, novel ICIs, cancer vaccines, chemoradiotherapy and targeted therapy small molecule inhibitors will continue to emerge in the future.
The future of tumor immunotherapy lies in a truly patient-oriented, personalized combination therapy approach.
references:
1. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021; 14: 156.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.