Half a tablet of tamoxifen a day can prevent breast cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Half a tablet of tamoxifen a day can prevent breast cancer
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Half a tablet of tamoxifen a day can prevent breast cancer 丨 10-year follow-up results.
Breast intraepithelial neoplasia, including atypical ductal hyperplasia (ADH), lobular carcinoma in situ (LCIS), and ductal carcinoma in situ (DCIS). Compared with the general population, the risk of invasive breast cancer is 5 to 10 times higher.
Previous studies have confirmed that standard doses of tamoxifen (20 mg/d) can prevent breast cancer in high-risk women, such as women with ADH and LCIS, but tamoxifen can cause serious complications such as venous thromboembolism and endometrial cancer. Toxicity limits its wide application.
Another study showed that compared with 20 mg/d tamoxifen, 5 mg/d tamoxifen had no significant difference in inhibiting the proliferation of breast cancer and normal endometrial tissue.
Therefore, the Italian Cancer Research Association and the European Oncology Institute of the University of Milan jointly initiated the TAM-01 study to compare the effectiveness of oral low-dose tamoxifen (5 mg/d) or placebo in the prevention of mammary intraepithelial neoplasia for 3 consecutive years. and safety, and published 5-year follow-up results in 2019.
The results showed that oral tamoxifen 5 mg per day reduced the incidence of invasive breast cancer or ductal carcinoma in situ by 52% compared with placebo, with similar adverse effects.
Recently, a research team led by Andrea DeCensi published a paper entitled “Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Recurrence in Breast Noninvasive Neoplasia: A 10-Year Follow-Up” in Journal of Clinical Oncology (JCO, IF: 50.7 ) of TAM-01 Study”, reporting the 10-year follow-up results of the TAM-01 trial, the results showed that continuous administration of low-dose tamoxifen (5 mg/d) for 3 years could significantly prevent non-invasive breast cancer recurrence without long-term adverse reactions .
 Screenshot of the homepage of the paper Source: Reference 2
Screenshot of the homepage of the paper Source: Reference 2
Research Background and Design
The study (TAM-01 study) is a multicenter, randomized controlled, III clinical trial. Between November 1, 2008, and March 31, 2015, 500 women aged ≤75 years with breast intraepithelial neoplasia were included, of which 20% had atypical breast ductal hyperplasia and 11% had breast lobular in situ 69% were hormone-sensitive or unknown ductal carcinoma in situ.
The mean age of the patients at the start of treatment was 54±9 years, and 58% were postmenopausal. Other general conditions and tumor characteristics are shown in the table below.
The enrolled patients were randomly assigned to the low-dose tamoxifen group (253 patients) or the placebo group (247 patients) , and received tamoxifen (5 mg/d) or placebo orally for 3 years, respectively. The primary endpoint was the incidence of invasive breast cancer or ductal carcinoma in situ.
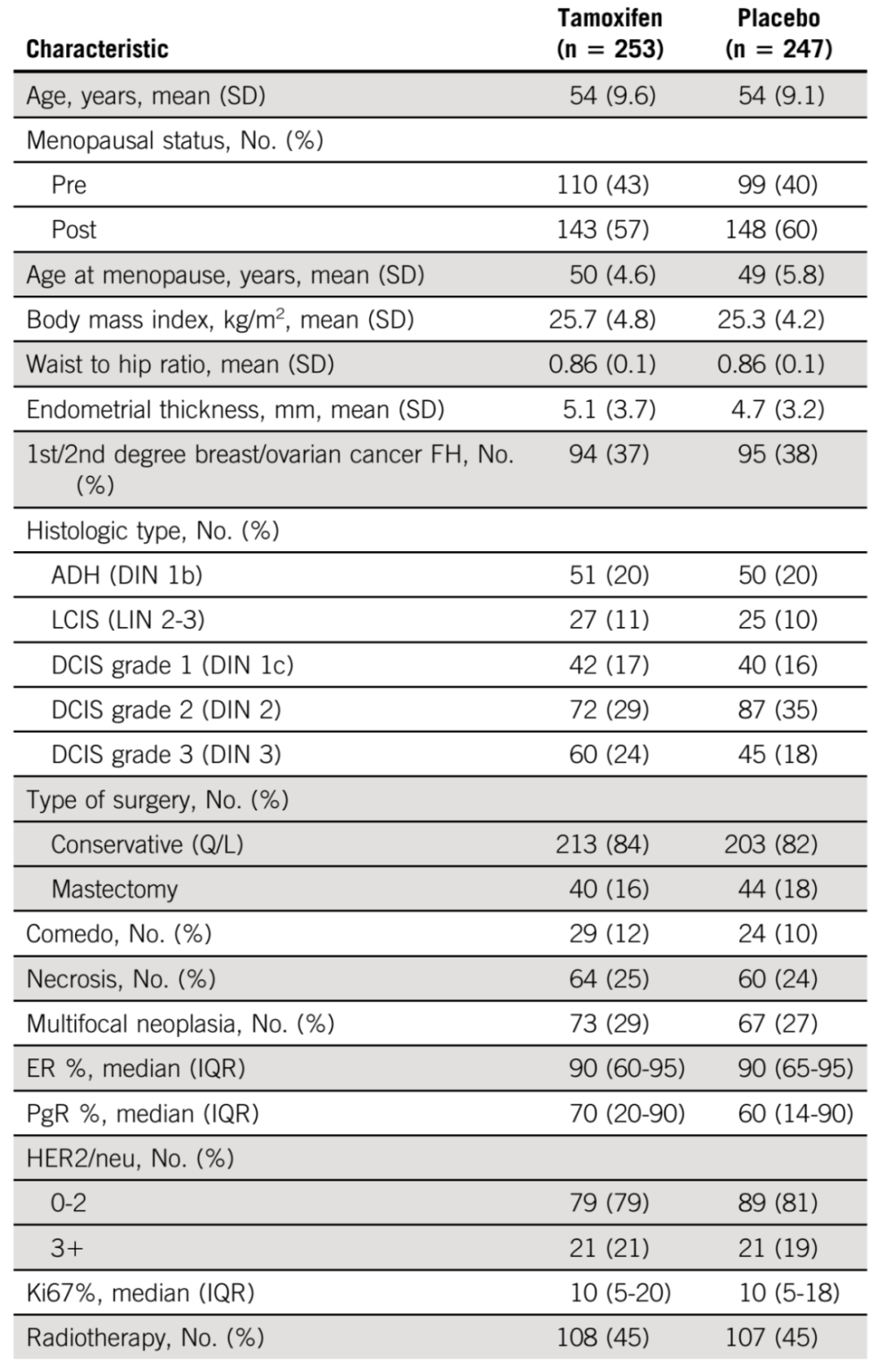
Patient profile and tumor characteristics at baseline Source: Reference 2
Research result
At a median follow-up of 9.7 years, 66 breast cancers were diagnosed, 15 of which were carcinoma in situ and 51 of which were invasive. Most recurrences were invasive (77%) and ipsilateral (59%).
Among patients diagnosed with breast cancer, 25 were in the tamoxifen group and 41 in the placebo group. Among the patients diagnosed with contralateral breast cancer, 6 were in the tamoxifen group and 16 in the placebo group.
Compared with the placebo group, the risk of breast cancer in the tamoxifen group was significantly reduced by 42% (annual incidence rate: 11.3‰ vs 19.5‰, hazard ratio: 0.58, P = 0.03), and the risk of contralateral breast cancer was significantly reduced by 64% ( Hazard ratio: 0.36, P = 0.025).
These benefits were also seen in a subgroup analysis, with a significant 50% reduction in recurrence in the tamoxifen group compared with placebo in the DCIS cohort (hazard ratio: 0.50, P = 0.02).
No significant differences in tumor stage and biological behavior occurred in the tamoxifen group compared with the placebo group. For every 1 case of breast cancer prevented by tamoxifen, the number of people who need to be treated is 22 cases in 5 years and 14 cases in 10 years.
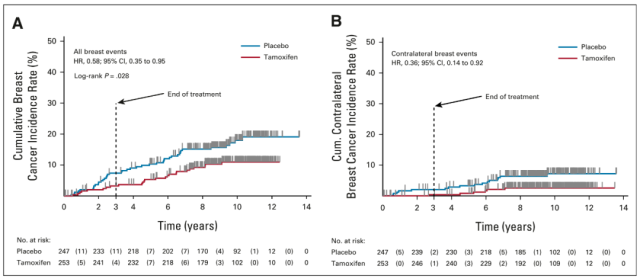 A Cumulative incidence of breast cancer B Cumulative incidence of contralateral breast cancer Source: Reference 2
A Cumulative incidence of breast cancer B Cumulative incidence of contralateral breast cancer Source: Reference 2
In terms of adverse events, there were 35 serious adverse events in the tamoxifen group (including one case of deep vein thrombosis and one case of stage I endometrial cancer) and 22 in the placebo group (including one case of pulmonary embolism).
There was no significant difference in the number of cancers other than breast cancer (16 [6.4%] vs 9 [3.7%]). Endometrial polyps occurred in 8.0% of the tamoxifen group and 5.3% of the placebo group (P = 0.28).
There were 10 deaths during the study period, 6 in the tamoxifen group and 4 in the placebo group.
Causes of death were breast cancer (1 vs 2) and other causes (5 vs 2) in the tamoxifen and placebo groups, respectively. There were no treatment-related deaths.
Overall, there was no significant difference in the incidence of serious adverse events between the tamoxifen group and the placebo group, and all treatment-related deaths occurred in both groups.
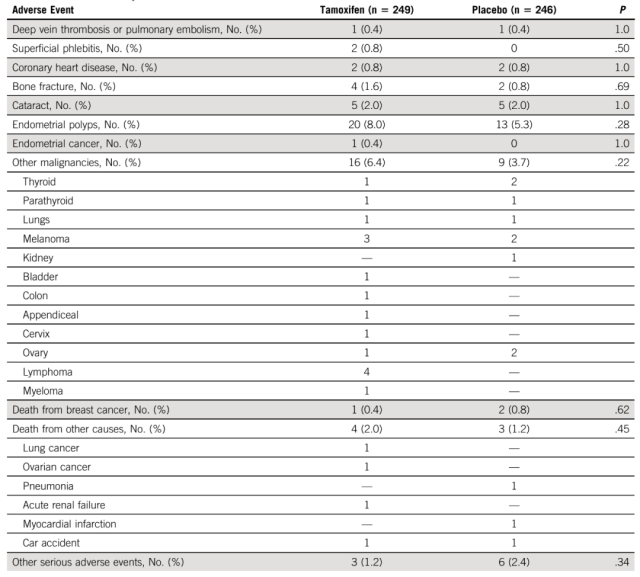 Adverse Event Comparison Chart Source: Reference 2
Adverse Event Comparison Chart Source: Reference 2
Discussion
In the initial report in 2019, it was described that taking tamoxifen 5 mg/d for 3 years after the diagnosis of intraepithelial neoplasia can significantly reduce the risk of breast cancer recurrence by 52%.
After nearly 10 years of follow-up, the incidence of invasive breast cancer continued to decline, suggesting that even at low doses, the benefit of tamoxifen persisted for at least 7 years after cessation of treatment.
In the NSABP P-1 trial, standard-dose tamoxifen (20 mg/d) tripled the risk of deep vein thrombosis and pulmonary embolism, increased the incidence of stage I endometrial cancer (2.24/1000 person-years), Endometrial polyps and cataracts occur frequently. In this study, low-dose tamoxifen did not increase serious adverse events.
Additionally, the low risk of breast cancer death 10 years after enrollment (3/500, 0.6%) suggests a favorable prognosis in this cohort and supports the emerging trend towards de-escalation of treatment for DCIS.
The favorable risk-benefit ratio makes low-dose tamoxifen an effective option in different situations.
First, in the adjuvant treatment of postoperative radiotherapy for DCIS. In this case, preventing any ipsilateral recurrence will also avoid complications associated with previously irradiated breast reconstruction.
Second, prophylaxis in women with high-risk lesions, including atypical ductal hyperplasia, atypical lobular hyperplasia, and lobular carcinoma in situ.
In this trial, a significant 64% reduction in the incidence of contralateral breast cancer was observed in the tamoxifen group, suggesting that low-dose tamoxifen has a strong potential for primary prevention in high-risk healthy women.
However, the study has some limitations:
① There is no comparison with the standard dose of 20 mg/d.
② There is a lack of 5 mg tamoxifen tablets in the market.
In conclusion, this study provides strong support for the use of low-dose tamoxifen after a diagnosis of noninvasive breast cancer and opens the door for new research into the primary prevention of breast cancer in high-risk women.
knowledge development
In 2022, the Breast Cancer Professional Committee of the Chinese Research Hospital Association released the “Expert Consensus on the Prevention of Female Breast Cancer in China 2022 Edition”. The consensus provided corresponding guidance on drug prevention. The expert group suggested:
1. Under the premise of sufficient notification, selective estrogen receptor modulators (such as tamoxifen, raloxifene, etc.) can be taken for breast cancer high-risk groups to prevent the occurrence of breast cancer.
2. Postmenopausal high-risk groups of breast cancer can use aromatase inhibitors (such as anastrozole) prophylactically, but bone mineral density and blood lipid monitoring should be carried out regularly.
The experts recommend the definition of high-risk groups for breast cancer:
①A clear genetic predisposition to breast cancer, that is, a first-degree relative with breast or ovarian cancer before the age of 50, and ≥2 second-degree relatives with breast or ovarian cancer; a first-degree relative with a BRCA mutant gene;
②Patients with atypical hyperplasia or lobular carcinoma in situ confirmed by breast biopsy;
③A history of chest radiotherapy before the age of 30;
④High-risk groups assessed by the Gail model.
references:
1、DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J Clin Oncol. 2019;37(19):1629-1637. doi:10.1200/JCO.18.01779
2、Lazzeroni M, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Recurrence in Breast Noninvasive Neoplasia: A 10-Year Follow-Up of TAM-01 Study [published online ahead of print, 2023 Mar 14]. J Clin Oncol. 2023;JCO2202900. doi:10.1200/JCO.22.02900
3. Expert Consensus on Prevention of Female Breast Cancer
Half a tablet of tamoxifen a day can prevent breast cancer
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



