Why did the Alzheimer’s drug candidate BACE1 inhibitor fail?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Why did the Alzheimer’s drug candidate BACE1 inhibitor fail?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Why did the Alzheimer’s drug candidate BACE1 inhibitor fail? Scientists have found the mechanism by which it promotes cognitive decline!
Alzheimer’s disease (AD) is an important factor leading to dementia. With the aging of the population, AD has become an imminent crisis.
Classical theory holds that Aβ deposition is one of the pathological causes of AD, so a variety of drugs have been developed specifically. β-secretase BACE1 is one of the catalytic enzymes that convert amyloid precursor protein (APP) into Aβ, and inhibiting the activity of BACE1 can reduce the production of Aβ protein.
A number of BACE1 inhibitors have , Most drugs stop in phase 2/3 clinical trials. It is unclear why BACE1 inhibitors cause cognitive decline in patients.
Recently, a research team led by Stefan F. Lichtenthaler of the German Center for Neurodegenerative Diseases published the latest research results on Molecular Neurodegeneration [4], revealing the unknown new function of BACE1.
They found that in nonhuman primates as well as humans, BACE1 cleaves the IL-6 receptor beta subunit (gp130), thereby affecting IL-6 signaling in neurons . Since soluble gp130 (sgp130) acts as a buffer system in the healthy state, preventing the adverse effects of excessive IL-6 signaling on neurons, long-term inhibition of BACE1 makes the adverse effects of IL-6 signaling more severe in treated individuals. sensitive .

Screenshot of paper home page
Because BACE1 inhibitors with different molecular structures can cause cognitive decline, and these drugs can inhibit the activity of BACE1 in the central nervous system by more than 75%, the Lichtenthaler team guessed that this may be due to the excessive inhibition of BACE1 on one or a certain Cleavage of several substrates, causing neurological side effects.
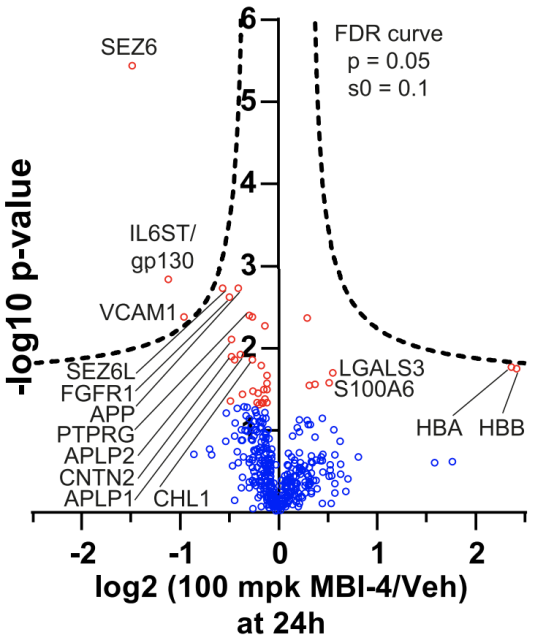
In order to find proteins that might be cleaved by BACE1, they collected cerebrospinal fluid samples from three rhesus monkeys before and after oral administration of the BACE1 inhibitor MBI-4, and used mass spectrometry to find differential proteins.
They found that the abundance of SEZ6, IL6ST (gp130), and the extracellular domain of VCAM1 in CSF were significantly reduced in a dose-dependent manner after administration of BACE1 inhibitors .
Among them, SEZ6 is a known substrate of BACE1; IL6ST is a cytokine receptor gp130, and existing studies suggest that it may be a substrate of BACE1 [5,6].
To further confirm the above findings, they conducted a separate experiment, administered the BACE1 inhibitor verubecestat, which is currently in clinical trials, to detect changes in the protein in the cerebrospinal fluid of macaques.
The results showed that SEZ6, CACHD1, LRRN1, L1CAM, and sgp130 in cerebrospinal fluid showed a dose-dependent reduction after administration of verubecestat.
The above two experiments showed that inhibition of BACE1 activity can reduce sgp130, suggesting that gp130 is a potential BACE1 substrate.
gp130 may be a substrate of BACE1
In order to explore whether the above phenomenon also exists in humans, the Lichtenthaler team collected cerebrospinal fluid samples from healthy young people before and after taking the BACE1 inhibitor verubecestat, and detected the levels of SEZ6 and sgp130 in the cerebrospinal fluid by WB and ELISA.
The results showed that after taking verubecestat, the levels of SEZ6 and sgp130 in human cerebrospinal fluid were significantly reduced.
These data suggest that SEZ6 and sgp130 may serve as markers of pharmacodynamic activity of BACE1.
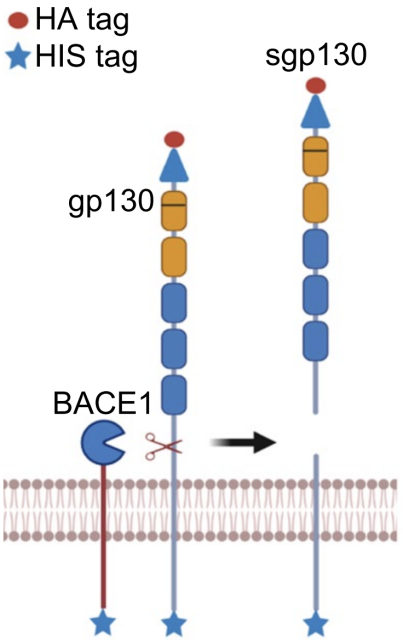
Now that data from non-human primates and human volunteers suggest that gp130 may be a substrate of BACE1, the next step is to confirm whether BACE1 can actually cleave gp130.
Through in vitro experiments, Lichtenthaler’s team directly confirmed that BACE1 can indeed cleave membrane-bound gp130 to generate sgp130.
Experiments based on primary mouse neurons also showed that BACE1 can indeed cleave gp130 to generate sgp130 in living neurons.
Cleavage of gp130 by BACE1 in vitro
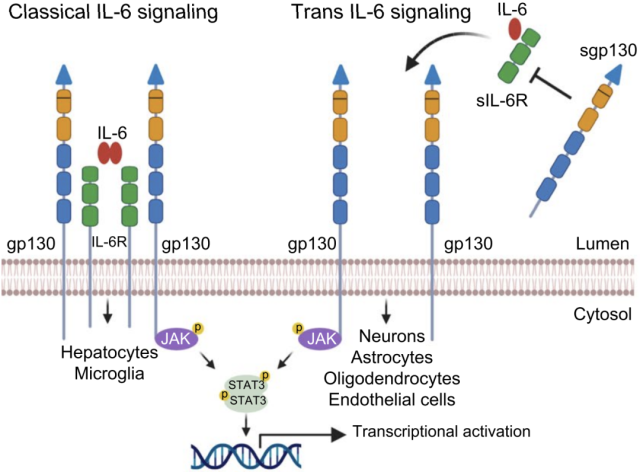
Since gp130 mediates the downstream signal transduction of IL-6, and sgp130 can inhibit the trans-signaling of IL-6, the Lichtenthaler team wondered whether inhibiting the activity of BACE1 would affect the neuronal response to IL by affecting the abundance of sgp130. -6 response.
To this end, they performed experiments using primary mouse neurons, stimulated the neurons with IL-6 and IL6R complex (H-IL-6), and inhibited the activity of BACE1 by adding C3.
The results showed that after inhibiting BACE1, the phosphorylation level of STAT3 downstream of IL-6 was significantly increased.
This may be due to the increase of gp130 on the cell membrane after inhibition of BACE1 activity, thus enhancing the downstream signal transduction of IL-6.
If H-IL-6 was incubated with sgp130 for a period of time and then added to the culture medium, the phosphorylation level of STAT3 in neurons was significantly reduced, indicating that sgp130 can compete with membrane-bound gp130 for binding to H-IL-6 , Thereby preventing neurons from responding to IL-6 stimulation .
In the case of excitotoxicity and lack of growth factors, gp130 signaling has a protective effect on neurons, so the research team used incomplete medium to culture mouse primary neurons, and compared the addition or absence of BACE1 inhibitor C3, Protective effect of H-IL-6 on neurons.
The results showed that inhibiting BACE1 increased the sensitivity of neurons to H-IL-6, allowing more neurons to survive.
Based on the above data, inhibiting the activity of BACE1 can not only reduce the cleavage of gp130 and enhance the ability of neurons to transduce IL-6 signals downstream, but also reduce the production of sgp130 and reduce the inhibition of IL-6 trans-signaling.
Both of these effects can increase the sensitivity of neurons to IL-6 signaling.
However, IL-6 is a double-edged sword for the nervous system. Although IL-6 can promote neurogenesis and promote neuronal survival during normal development, excessive activation of IL-6 signaling can cause damage to the nervous system. [7].
Considering that the concentration of IL-6 in the serum of AD patients is higher than that of healthy people, inhibiting BACE1 to increase the sensitivity of neurons to IL-6 may not necessarily be a good thing for AD patients.
Inhibition of BACE1 affects IL-6 trans-signaling in neurons
Overall, this study is the first to clearly demonstrate that BACE1 cleaves gp130 to generate sgp130, and that inhibition of BACE1 activity results in reduced cleavage of gp130 on neurons by BACE1, increased gp130 content on neurons and decreased sgp130 content in the brain , which causes neurons to be more sensitive to IL-6 signaling .
Considering that existing studies have shown that excessive activation of IL-6 signaling is associated with cognitive decline in AD [7], therefore, strong inhibition of BACE1 activity may enhance the sensitivity of neurons to IL-6 signaling, while lead to cognitive impairment in patients.
This discovery by the Lichtenthaler team has great significance for the clinical development of BACE1 inhibitors.
For example, the degree of reduction of sgp130 in cerebrospinal fluid or blood in phase 3 clinical trials can be combined with the extent of cognitive decline of patients to find a safe range of sgp130 content and reduce the dosage of BACE1 inhibitors in a targeted manner .
Moreover, preclinical studies have shown that reducing the activity of BACE1 by 50% or less can still reduce the increase of amyloid plaques [9].
So it might be possible to find a drug regimen that reduces amyloid plaques while avoiding the serious side effects of treatment.
references:
1. McDade E, Voytyuk I, Aisen P , Bateman RJ, Carrillo MC, De Strooper B, et al The case for low-level BACE1 inhibition for the prevention of Alzheimer disease. Nat Rev Neurol. 2021;17(11):703 –14.
2. Henley D, Raghavan N, Sperling R, Aisen P , Raman R, Romano G. Preliminary results of a trial of Atabecestat in preclinical Alzheimer’s disease. N Engl J Med. 2019;380(15):1483–5.
3. Wessels AM, Lines C, Stern RA, Kost J, Voss T, Mozley LH, et al Cognitive outcomes in trials of two BACE inhibitors in Alzheimer’s disease. Alzheimer-mers Dement. 2020;16(11):1483–92.
4. Müller SA, Shmueli MD, Feng X, et al. The Alzheimer’s disease-linked protease BACE1 modulates neuronal IL-6 signaling through shedding of the receptor gp130. Mol Neurodegener. 2023;18(1):13. Published 2023 Feb 21 .doi:10.1186/s13024-023-00596-6
5. Stutzer I, Selevsek N, Esterhazy D, Schmidt A, Aebersold R, Stoffel M. Systematic proteomic analysis identifies beta‑site amyloid precursor protein cleaving enzyme 2 and 1 (BACE2 and BACE1) substrates in pancreatic beta‑cells. J Biol Chem . 2013;288(15):10536–47.
6. Hemming ML, Elias JE, Gygi SP , Selkoe DJ. Identification of β‑secretase (BACE1) substrates using quantitative proteomics. PLoS One.2010;4(12):e8477.
7. Lyra ESNM, Goncalves RA, Pascoal TA, Lima‑Filho RAS, Resende EPF , Vieira ELM, et al Pro‑inflammatory interleukin‑6 signaling links cogni‑ tive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl Psychiatry; 12021. (1):251
8. McDade E, Voytyuk I, Aisen P , Bateman RJ, Carrillo MC, De Strooper B, et al The case for low‑level BACE1 inhibition for the prevention of Alzheimer disease. Nat Rev Neurol. 2021;17(11):703 –14
Why did the Alzheimer’s drug candidate BACE1 inhibitor fail?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



