Alzheimer’s disease: Astrocyte reactivity is a key link in Aβ-driven tau pathology
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Alzheimer’s disease: Astrocyte reactivity is a key link in Aβ-driven tau pathology
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Alzheimer’s disease: Astrocyte reactivity is a key link in Aβ-driven tau pathology.
“Nature Medicine”: A major breakthrough in the mechanism of Alzheimer’s disease! Scientists discover that astrocyte reactivity is a key link in Aβ-driven tau pathology 丨 Scientific Discovery
For example, one of the main pathological manifestations of AD, β-amyloid (Aβ) deposition does not necessarily lead to tau pathology and clinical progression . Numerous biomarker studies have shown that in cognitively unimpaired (CU) individuals, Aβ precedes tau deposition, which is closely related to the development of cognitive symptoms. However, some individuals can continue to maintain cognition in the presence of Aβ pathology, and eventually do not develop AD .
Which part of it have we overlooked?
On May 30, “Nature Medicine” published the latest research results of the research team of the University of Pittsburgh. Scientists analyzed biomarker data from more than 1,000 cognitively unimpaired participants in three cohorts and uncovered an important fact—whether Aβ pathology leads to tau pathology depends on astrocyte status .
Aβ burden was functionally related to plasma phosphorylated tau and tau tangles only in astrocyte-reactive (Ast) individuals.
The findings of this study suggest that astrocyte reactivity is an important upstream event linking Aβ to the development of tau pathology, and that the inclusion of astrocyte reactivity markers in preclinical AD classification may be useful in the search for AD interventions. Windows are important.

The fact that Aβ does not necessarily cause tau pathology and cognitive problems suggests that there are other processes that can trigger Aβ biotoxicity in the early stages of disease.
Autopsy studies have shown that astrocyte reactivity (Ast) is a common neuropathology in cognitively intact individuals and, along with cortical Aβ plaques, is one of the earliest abnormal pathologies in AD.
Studies have shown that astrocyte reactivity is critical for triggering Aβ-induced tau phosphorylation, and inhibition of astrocyte reactivity can attenuate tau pathology ; glial fibrillary acid protein (GFAP)-positive astrocytes Plastid cells are able to internalize tau and may contribute to tau replication.
These findings support a link between Aβ, astrocyte reactivity, and tau pathology.
To further test this hypothesis, the researchers analyzed the relationship between Aβ burden, phosphorylated tau/tau tangles and Ast using data from TRIAD, Pittsburgh cohort and MYHAT 3 cohort studies.
From 3 cohorts, the researchers selected a total of 1016 cognitively unimpaired individuals with a mean age of 69.6±8.9 years and a CDR score of 0. Plasma GFAP correlates with cerebrospinal fluid (CSF) levels, so positive astrocyte reactivity can be judged based on plasma GFAP levels.
In addition, only p-tau181 is the only phosphorylated tau protein marker with data retained in the three cohorts, so p-tau181 was used as the main analysis object.
Overall, participants classified as Aβ + /Ast + had higher plasma p-tau181, p-tau231, and p-tau217 compared to other groups . There was no difference in Aβ levels between Aβ + /Ast + and Aβ + /Ast – groups.
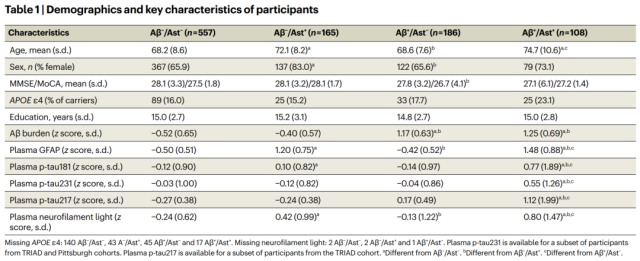 Basic information of participants
Basic information of participants
Interesting results emerge from Z-score and Cohen’d analysis. Aβ burden was significantly associated with plasma p-tau181 levels only in Ast-positive CU individuals ; in Ast-negative cases, Aβ had almost negligible effect on tau phosphorylation.
The same analysis was performed in the 3 cohorts, and the results were also consistent, and no effect of APOE4 status was observed.
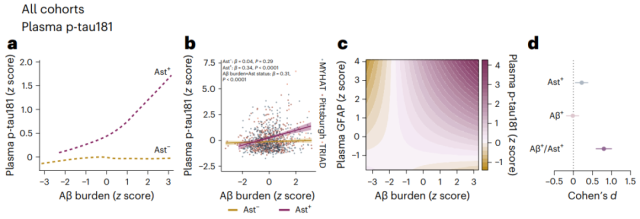 The relationship between Aβ and phosphorylated tau is driven by Ast
The relationship between Aβ and phosphorylated tau is driven by Ast
The researchers also analyzed the available p-tau231 and p-tau217 data separately, and found that the association of Aβ burden with phosphorylated tau was only observed in Ast-positive individuals .
Notably, Ast status did not affect the association between Aβ load and neurofilament light chain levels in any cohort, suggesting that astrocyte reactivity is involved in the impact of Aβ pathology on tau pathology, but not in neurodegeneration .
The researchers further analyzed gender differences in this process. Interestingly, Aβ burden was more correlated with plasma p-tau in male Ast-positive individuals .
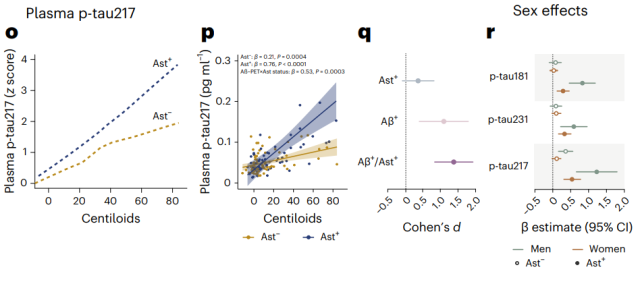 Other p-tau showed similar effects
Other p-tau showed similar effects
Aβ is more strongly correlated with tau in males (right)
The researchers also observed a similar association between Aβ burden and tau tangle deposition in Ast-positive individuals , and the affected brain regions were only those associated with early Braak grading.
Annual accumulation of tau tangles detected by PET was higher in Ast-positive individuals and could be predicted by baseline Aβ load.
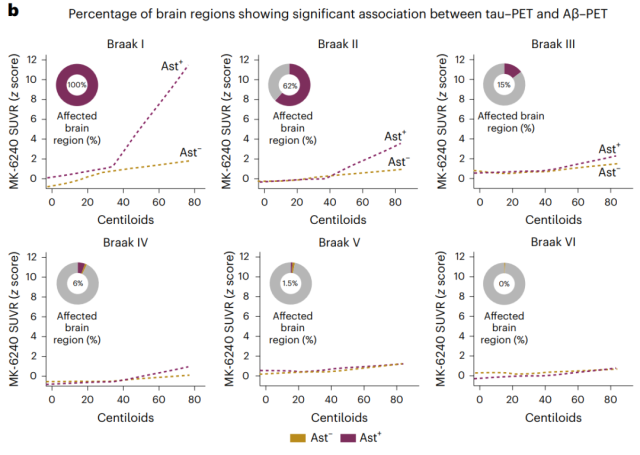 Percent brain area-wide with significant association of tau tangles with Aβ load at each Braak level
Percent brain area-wide with significant association of tau tangles with Aβ load at each Braak level
These results support previous biomarker studies showing that astrocyte reactivity is an early upstream event that precedes tau pathology.
The researchers pointed out that incorporating Ast into the current biomarker model and using Aβ/Ast/p-tau as the classification standard for preclinical AD should provide a more refined intervention window for clinical trials, and target Aβ and astrocytosis.
The drug combination of glial cells can also lead to new AD prevention/treatment options.
References:
[1] Bellaver, B., Povala, G., Ferreira, PCL et al. Astrocyte reactivity influences amyloid-β effects on tau pathology in preclinical Alzheimer’s disease. Nat Med (2023). https://doi.org/10.1038/ s41591-023-02380-x
Alzheimer’s disease: Astrocyte reactivity is a key link in Aβ-driven tau pathology
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



