Alzheimer’s disease: Plasma p-tau217 predicts pathological and cognitive changes
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Alzheimer’s disease: Plasma p-tau217 predicts pathological and cognitive changes
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Alzheimer’s disease: Plasma p-tau217 predicts pathological and cognitive changes.
AD: Scientists find that plasma p-tau217 predicts pathological and cognitive changes 8 years later in patients with autosomal dominant Alzheimer’s disease!
PET imaging of tau protein neurofibrillary tangles in vivo is beneficial for the research, diagnosis, treatment and monitoring of Alzheimer’s disease (AD) [1].
Studies have shown that tau protein in cerebrospinal fluid (CSF) can also be used as an early and specific indicator of AD pathology [2]. However, sensitive, economical and minimally invasive AD biomarkers are also urgently needed.
Phosphorylated tau protein (p-tau) assays in plasma are less costly and less invasive than PET or CSF markers.
Moreover, plasma p-tau217 is elevated from preclinical to clinical disease stages, indicating that plasma p-tau217 has better early diagnostic ability [3].
In addition, studies have shown that plasma p-tau217 is associated with increased tau PET measurements in the olfactory cortex and medial temporal lobe over the following 1 to 2 years [4-5].
If plasma p-tau217 can predict tau PET in a longer period of time in the future, it is beneficial to determine plasma p-tau217 as an early marker of AD pathology, and it is also beneficial to recruit patients in clinical trials at an early stage.
Recently, the team of Yakeel T. Quiroz from Massachusetts General Hospital affiliated to Harvard Medical School published an important study in the famous journal Alzheimer’s & Dementia [6].
The research team found that plasma p-tau217 was elevated in ADAD mutation ( PSEN1 E280A) carriers with unimpaired cognitive function compared with autosomal dominant Alzheimer’s disease (ADAD) mutation non-carriers .
They also found that higher baseline plasma p-tau217 levels, which were associated with higher amyloid (Aβ) levels and tau PET pathology burden after a mean follow-up of 7.61 years, were also associated with poorer cognitive function .
This further proves that plasma p-tau217 can be used as a biomarker to detect early AD and predict its progression, and plasma p-tau217 will have broad application prospects in clinical practice and trials.

Screenshot of paper homepage
Next, let’s take a look at how this research was carried out.
In this cohort study, 24 PSEN1 E280A mutation carriers (23 Aβ pathologically positive) and 20 age- and education-matched non-carriers from the same family were enrolled in COLBOS at Massachusetts General Hospital (Columbia-Boston) Longitudinal biomarker study cohort.
All included participants had normal cognitive function at baseline.
Researchers performed blood sampling at baseline and completed neuroimaging and cognitive memory assessments at the end of follow-up (average follow-up interval, 7.61 years).
The researchers first compared the group differences in the basic characteristics of the participants at baseline (Table 1), and found that the plasma p-tau217 levels of mutation carriers were higher than those of non-carriers (W=122, P=0.005) .
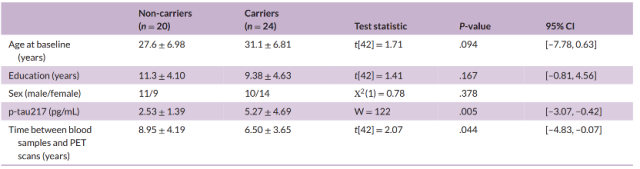
Table 1 Baseline characteristics of the included population
They then assessed group differences in participants’ neuroimaging and cognitive memory at the end of follow-up (Table 2) and found that of the 24 carriers, 18 were cognitively unimpaired at the end of follow-up and 6 developed Mild Cognitive Impairment (MCI).
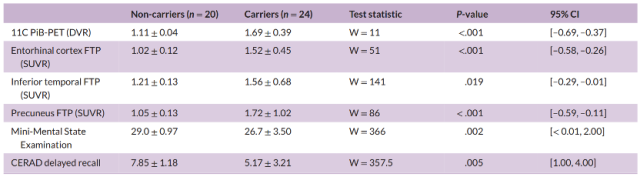
Table 2 Follow-up neuroimaging and cognitive data
Subsequently, to assess the utility of plasma p-tau217 as an early AD biomarker, we examined the association of plasma p-tau217 with various markers of AD in the entire population.
They found that older age at baseline was associated with higher plasma p-tau217 levels (Fig. 1A), and that higher plasma p-tau217 at baseline was associated with lower MMSE scores (Fig. 1B) and delayed recall scores (Fig. Figure 1C) related.
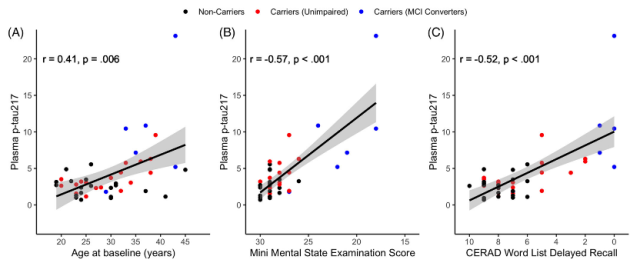
Figure 1 Correlation of plasma p-tau217 with age and cognitive function in the whole population
They also found that higher baseline plasma p-tau217 was associated with higher cortical Aβ PET measurements at the end of follow-up (Fig. 2A), and also with higher entorhinal cortex (Fig. 2B), inferior temporal cortex (Fig. 2C ) and tau PET measurements in the precuneus (Fig. 2D) .
In addition, the researchers also performed the above analysis in mutation carriers and non-carriers separately, and found that only mutation carriers had statistically significant correlations between plasma p-tau217 and age, cognitive function, and PET pathology.
After adjusting for age, sex, and follow-up time, the researchers observed consistent results across the population: Baseline plasma p-tau217 was inversely associated with cognitive levels at the end of follow-up, correlated with cortical Aβ PET measurements as well as with entorhinal cortex, There was a positive correlation between tau PET measurements in the infratemporal cortex and precuneus. They also performed the above corrections in mutation carriers and non-carriers, but did not get consistent results, which may be the reason for the small sample size.
They also found no significant association between plasma p-tau217 and tau PET when Aβ was used as a covariate , but the inverse association with cognition remained significant.
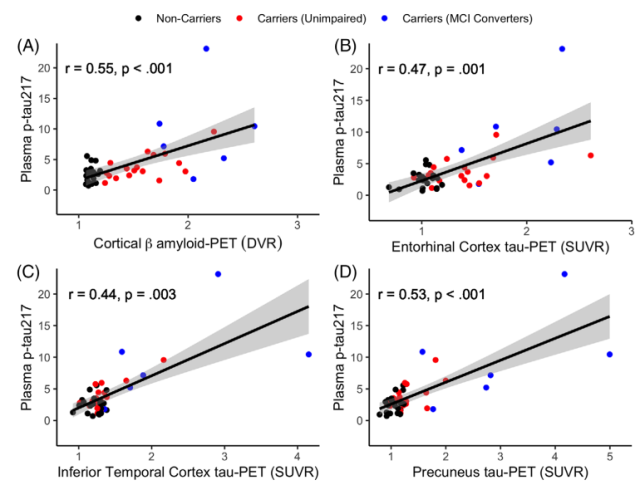
Figure 2 Correlation between plasma p-tau217 and PET pathology in the whole population
Finally, we explored the relationship between plasma p-tau217 and whole brain Aβ and tau PET in mutation carriers and cognitively normal mutation carriers at the end of follow-up.
They found that in mutation carriers, plasma p-tau217 was positively correlated with Aβ burden in the frontal, lateral temporal, parietal, and posterior splenium cortices; the correlation between plasma p-tau217 and tau PET was highest in the temporal and parietal lobes Strong, consistent with the known anatomy of early tau accumulation in mutation carriers (Figure 3A).
When we restricted our analysis to cognitively normal mutation carriers, we found that plasma p-tau217 was predominantly associated with Aβ and tau PET in the left lateral temporal cortex (Fig. 3B).
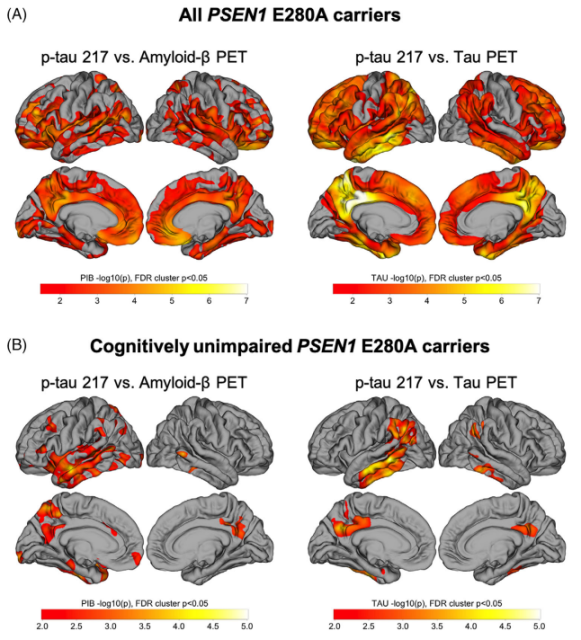
Figure 3 Relationship between plasma p-tau217 and whole brain Aβ and tau PET in mutation carriers and cognitively normal mutation carriers after follow-up
After adjusting for age, sex, and follow-up time, the researchers found that these results were attenuated in mutation carriers, but the relationship between p-tau217 and tau PET was similar to the unadjusted results.
They also performed this adjustment in cognitively normal mutation carriers at the end of follow-up and found that the adjusted results were not statistically significant.
Likewise, they included cortical Aβ as a covariate and found that the association of the results was attenuated in mutation carriers, whereas the results were not statistically significant in cognitively unimpaired carriers.
Overall, this study demonstrates the correlation between plasma p-tau217 and PET pathology and cognitive function indicators, suggesting that baseline plasma p-tau217 predicts subsequent Aβ and tau levels and cognitive function in PSEN1 E280A carriers. performance .
The results of this study strongly prove that plasma p-tau217 can be used as a minimally invasive diagnostic marker for early AD.
Due to the low cost of measurement, small trauma, and easy to carry out in the population, it is expected to promote the early screening and diagnosis of AD.
references:
[1]. Chandra A, Valkimadi P, Pagano G, Cousins O, Dervenoulas G, Politis M. Applications of amyloid, tau, and neuroinflammation PET imaging to Alzheimer’s disease and mild cognitive impairment. Hum Brain Mapp. 2019;40(18) :5424-5442.
[2]. Bjerke M, Engelborghs S. Cerebrospinal fluid biomarkers for early and differential Alzheimer’s disease diagnosis. J Alzheimers Dis JAD. 2018;62(3):1199-1209.
[3]. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021);20( :739-752.
[4]. Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho-tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021;78(2):149-156.
[5]. Leuzy A, Smith R, Cullen NC, et al. Biomarker-based prediction of longitudinal tau positron emission tomography in Alzheimer disease. JAMA Neurol. 2022;79(2):149-158.
[6]. Aguillon, D, Langella, S, Chen, Y, et al. Plasma p-tau217 predicts in vivo brain pathology and cognition in autosomal dominant Alzheimer’s disease. Alzheimer’s Dement. 2022; 1- 10.
Alzheimer’s disease: Plasma p-tau217 predicts pathological and cognitive changes
(source: Internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided in medicaltrend.org is for informational purposes only and should not be considered as medical advice.



