Pfizer invests US$25 million for the CAR-T cell therapy of multiple myeloma
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Pfizer invests US$25 million for the CAR-T cell therapy of multiple myeloma
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Pfizer invests US$25 million for the CAR-T cell therapy of multiple myeloma.
Caribou, a CRISPR-based off-the-shelf cell therapy company, received $25 million investment from Pfizer.
In October 2017, the FDA approved the marketing of the first CAR-T cell therapy , and humans entered the era of cell therapy.
CAR-T cell therapy has achieved good clinical results in blood cancers. Since CAR-T cells are modified from the patient’s own T cells, they can function in the patient’s body for a long time without rejection.
Now, CAR-T cell therapy has become the standard treatment for children with B-cell acute lymphoblastic leukemia (B-ALL) .
However, genetically engineering a patient’s own T cells takes a long time, and some acute leukemia patients don’t have enough time to wait. In addition, many severe patients already do not have enough T cells to engineer.
Therefore, CAR-T constructed from T cells derived from healthy donors can avoid the above-mentioned problems and may develop “off-the-shelf” CAR-T products . But this requires additional genetic modification of the CAR-T cells to prevent graft-versus-host disease (GvHD) and the body’s rejection of the CAR-T cells .
Recently, Pfizer announced that it will provide Caribou Biosciences with a US$25 million equity investment to support its development of the CAR-T cell therapy for the treatment of multiple myeloma .

Caribou Biosciences was founded by Professor Jennifer Doudna , the 2020 Nobel Prize winner in Chemistry and one of the founders of CRISPR gene editing technology, and her student Rachel Haurwitz , who serves as the company’s CEO.
Caribou Biosciences is committed to developing the next generation of “off-the-shelf” CAR-T and CAR-NK cell therapies through CRISPR gene editing technology to solve the host immune system’s rejection of allogeneic cell therapy and benefit more cancer patients.
Caribou is reported to have invested the funds in the development of CB-011 therapy, an allogeneic CAR-T cell therapy, which is currently in a phase 1 clinical trial in patients with relapsed or refractory multiple myeloma. Caribou said that the therapy has been approved by the FDA as a fast track, and it is one of the first allogeneic CAR-T cell therapies to enter the clinic, aiming to improve anti-tumor activity through immune stealth strategies. The therapy targets BCMA and is designed to remove B2M proteins and insert B2M- HLA-E fusion proteins to prevent immune-mediated rejection .
Encouraged by Caribou ‘s chRDNA technology , the potential allogeneic cell therapy has become an off-the-shelf cancer treatment, Pfizer said . chRDNA, that is, CRISPR hybrid RNA-DNA technology, compared with CRISPR-Cas9, chRDNA has high specificity and lower off-target editing level, and can realize multiple gene editing, including multiple gene insertion.
On May 12, 2022, Caribou announced the data of the first human clinical trial. The spot CAR-T therapy CB-010 based on CRISPR gene editing developed by Caribou was effective in the treatment of relapsed and refractory B-cell non-Hodgkin’s lymphoma . Positive results in Phase 1 clinical trials. Among the 5 evaluable patients, the overall response rate (ORR) was 100%, and the complete response rate (CR) was 80%.
However, a month later, Caribou announced again that 50% of patients who received the above-mentioned off-the-shelf CAR-T cell therapy had their cancer relapse within 6 months after treatment, which also triggered doubts about the durability of the off-the-shelf CAR-T therapy. doubt .
In fact, the durability of the treatment effect has become an industry challenge for spot CAR-T. Previously, Allogene, CRISPR Therapeutics, Precision Biosciences and other companies had a considerable proportion of cancer patients relapse within 6 months after treatment.
Caribou said that it has begun to recruit cancer patients for dose-doubling clinical trials (up from the previous infusion of 40 million CAR-T cells to 80 million) . Doubling the dose of CAT-T cells can promote a more durable treatment response, Caribou said it is confident in the durability of the re-boosted therapeutic effect.
Currently, Caribou has 4 self-developed pipelines and 2 pipelines jointly developed with AbbVie .
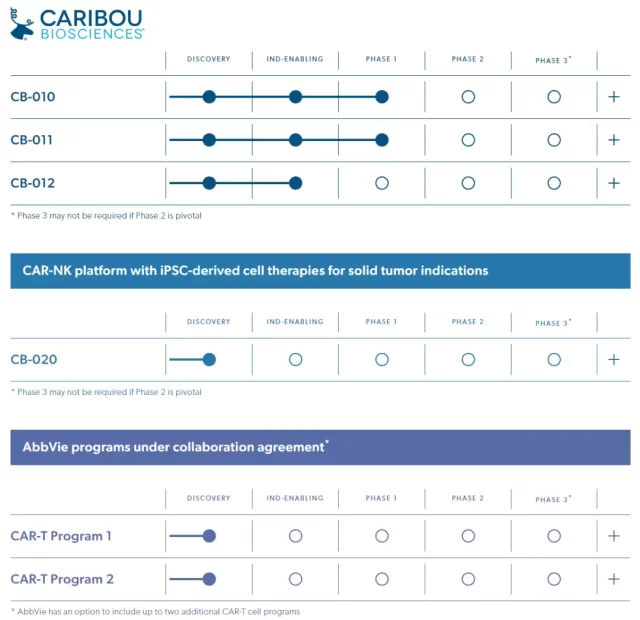
Among the self-developed pipelines, 3 are CAR-T therapies and 1 is CAR-NK therapy. The three CAR-T therapies are all targeting hematological malignancies, among which CB-010 and CB-011 are undergoing phase 1 clinical trials. CB-010 is a CD19-targeting CAR-T therapy for the treatment of B cell non-Hodgkin Chiggin’s lymphoma. CB-011 is a CAR-T therapy targeting BCMA for the treatment of multiple myeloma. CB-012 is undergoing clinical trial application, which is a CAR-T therapy targeting CD137 for the treatment of acute myeloid leukemia.
CB-020 is an iPSC-based CAR-NK cell therapy for the treatment of solid tumors, and specific target information will be announced in the fourth quarter of this year.
CB-010 uses the Cas9-chRDNA gene editing technology, and other pipelines use the Cas12a-chRDNA gene editing technology.
References :
https://www.cariboubio.com/
Pfizer invests US$25 million for the CAR-T cell therapy of multiple myeloma.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



