Chronic inflammation is a driver of p53 gene mutation cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Chronic inflammation is a driver of p53 gene mutation cancer
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Chronic inflammation is a driver of p53 gene mutation cancer
The p53 gene (TP53) has been one of the focuses of cancer research since its discovery in 1979.
Using p53 as a keyword to search in the Pubmed database, more than 100,000 articles can be found.
In various cancers, p53 is a tumor suppressor gene with a wide and powerful function, known as the “guardian of the genome”.
p53 is activated in response to various stress sources (such as DNA damage) in the cell.
The activation of p53 promotes DNA repair, or induces controlled death of abnormal cells, thereby preventing the occurrence and development of cancer.
Therefore, p53 gene mutations are very common in many cancers, and about half of cancer patients carry p53 gene mutations. The mutation of the p53 gene is an important driving force for the occurrence, development, drug resistance and poor prognosis of cancer.
Hematopoietic stem cells (HSCs) can differentiate into all types of blood cells, which is essential for maintaining a healthy blood system. Previous studies have shown that p53 gene mutations in HSCs are associated with cancer progression pathways that lead to acute myeloid leukemia (AML). But so far, the mechanism by which these p53 gene mutated blood stem cells expand and cause cancer is still unknown.
On September 4, 2023, researchers from Oxford University published a research paper titled: Single-cell multi-omics identifies chronic inflammation as a driver of TP53-mutant leukemic evolution in the journal Nature Genetics.
The study identified chronic inflammation as a driver of p53 gene mutation leukemic evolution by single-cell multi-omics, revealing a previously unknown effect of chronic inflammation on p53 gene mutated hematopoietic stem cells.

Image p53 gene mutation is the most common mutation gene in human cancers, and about half of cancer patients carry p53 gene mutations. In this study, the research team explored the relationship between leukemia and p53 gene mutations in hematopoietic stem cells.
The research team performed single-cell multi-omics analysis on hematopoietic stem/progenitor cells (HSPCs) from patients with myeloproliferative neoplasms transformed to p53 gene mutated secondary acute myeloid leukemia (sAML), confirming that chronic inflammation is an unrecognized factor influencing leukemia, which inhibits p53 wild-type HSPCs while enhancing the adaptive advantage of p53 gene mutated cells and promoting genetic evolution.
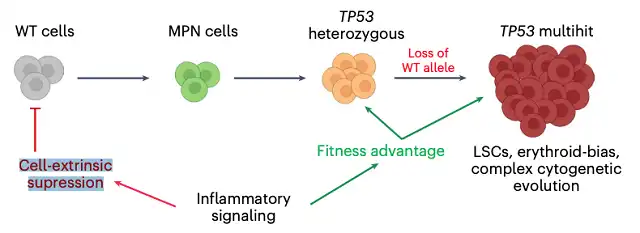
These findings will help to risk stratify, early detect and develop new treatment strategies for p53 gene mutated leukemia, and also have broad relevance to other cancer types due to the widespread presence of p53 gene mutations in cancer patients.
Hematopoietic stem cells and inflammation
The p53 gene is called the “guardian of the genome”, which is responsible for ensuring that cells do not carry genetic errors. Once errors occur in cells, p53 will initiate DNA repair mechanisms. When errors cannot be repaired, p53 will initiate programmed cell death, thereby preventing cell proliferation and preventing cancer occurrence and development.
Normally, when the body senses inflammation occurring, hematopoietic stem cells (HSCs) will differentiate into white blood cells. The produced white blood cells will help fight infection. However, this latest study shows that in leukemia patients with p53 gene mutations in hematopoietic stem cells, inflammation leads to selective expansion of p53 gene mutated cells. These hematopoietic stem cells cannot differentiate normally.
In patients with p53 gene mutations, stem cells cannot maintain their genome integrity. In this case, inflammation depletes normal hematopoietic stem cells in the bone marrow like a storm, while promoting the expansion of cells carrying p53 gene mutations, which eventually leads to aggressive cancer.
How do hematopoietic stem cells evolve into leukemia cells?
In this study, the research team used the TARGET-seq technique developed by the team before.
This technique can distinguish hematopoietic stem cells carrying p53 gene mutations with high resolution, as well as their behavior during patient disease progression.
The research team analyzed cells donated by patients and found that cells carrying p53 gene mutations from patients who were more likely to develop leukemia showed activation of genes associated with inflammatory processes.
Then, the research team confirmed in a mouse model that when mice suffered from inflammation, the number of cells carrying p53 gene mutations increased.
Next, the research team detected typical effects of inflammation in normal and mutant hematopoietic stem cells.
They found that hematopoietic stem cells carrying p53 gene mutations produced fewer white blood cells and were resistant to cell death, which was usually caused by inflammation.
This allowed mutant hematopoietic stem cells to “win the game”, resulting in an increase in the number and proportion of p53 gene mutated cells in the blood.
The study also found how blood stem cells maintain their genome integrity when stimulated by inflammation.
They found that p53 gene mutations changed the way cells responded to their DNA damage, making them unable to effectively repair these genetic errors, some of which helped them grow faster and promote cancer development.
Dr. Alba Rodriguez-Meira, the first author of the paper, said that overall, these findings provide valuable insights into how genetic defects and inflammation interact in the development of blood cancers.
Importantly, this study paves the way for early detection and development of new treatment methods for p53 gene mutated leukemia and many other cancer types, which will help improve the outcomes of cancer patients.
Professor Adam J. Mead, the corresponding author of the paper, said that he was very proud of this work, which illustrates how cutting-edge single-cell techniques can provide new insights into human diseases.
The connection between inflammation and genetic evolution in cancer has broad implications, and the next challenge is to identify how we can intervene in this process to more effectively treat or even prevent inflammation associated with cancer progression.
Chronic inflammation is a driver of p53 gene mutation cancer
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



