Immunomodulatory Mechanisms of Targeted Anticancer Drugs
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Immunomodulatory Mechanisms of Targeted Anticancer Drugs
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Immunomodulatory Mechanisms of Targeted Anticancer Drugs
Over the past three decades, extensive preclinical and clinical data in the field of cancer research have indicated that almost all targeted anticancer drugs exert some degree of immunostimulatory or immunosuppressive effects, influencing treatment outcomes.
The immunomodulatory activity of targeted anticancer drugs can arise from interactions with cancer cells and immune cells, altering their functions.
The general mechanisms of immunomodulation in targeted anticancer therapies can involve both direct (increasing specific effects) and indirect (reducing antagonistic effects) pathways.
For instance, these drugs may mediate immunostimulatory effects by promoting the secretion of pro-inflammatory cytokines or limiting the release or activity of immunosuppressive factors.
Furthermore, targeted anticancer drugs can induce highly immunogenic cancer cell death, initiating the so-called cancer-immunity cycle or selectively promoting the depletion of immunosuppressive cells, such as regulatory T (Treg) cells.
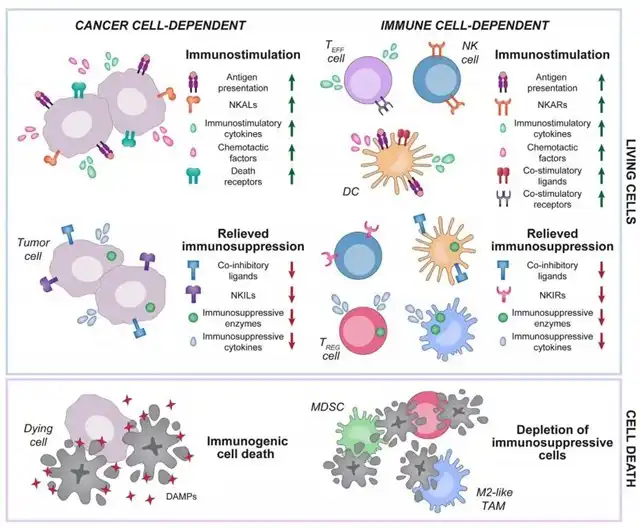
The ability of targeted anticancer drugs to mediate immunomodulation provides a robust theoretical foundation for developing combination therapies involving immunotherapy.
Below, we review the latest mechanistic progress in the primary immunomodulatory effects of FDA-approved and experimental targeted anticancer drugs, offering potential avenues for combining these drugs with immunotherapy to achieve enhanced clinical efficacy.
CDK Inhibitors
Cyclin-dependent kinases (CDKs) regulate cell cycle processes and other cellular functions. CDK4/CDK6 inhibitors, approved for HR+ breast cancer patients, have shown evidence of immunomodulation beyond inhibiting malignant cell proliferation.
CDK4/CDK6 inhibition can enhance immunostimulatory effects by exposing MHC I molecules on tumor cell surfaces and promoting the secretion of pro-inflammatory cytokines, such as type III interferons and CC chemokine ligand 5 (CCL5). Moreover, CDK4/CDK6 inhibitors directly interact with immune cells, mediating various immunostimulatory effects, including activation of effector T (TEFF) cells, inhibition of immunosuppressive regulatory Treg cells through DNA methyltransferase 1 (DNMT1) suppression, and cell cycle blockade via CDK inhibitor 1A (CDKN1A).
The immunostimulatory activity of CDK4/CDK6 inhibitors can be counteracted by their ability to upregulate the immunosuppressive molecule CD274 (PD-L1). Therefore, combining CDK4/CDK6 inhibitors with immune checkpoint inhibitors (ICIs) targeting PD-L1 or its receptor PD-1 is considered an ideal partnership.
Additionally, CDK4/CDK6 inhibitors can synergize with other targeted anticancer drugs for enhanced immunostimulation and superior efficacy. For example, the MEK inhibitor trametinib, combined with palbociclib, induces SASP-dependent vascular reactions, facilitating tumor infiltration by immune effector cells.
KRAS and PI3K Inhibitors
Some tumors are driven by mutations in KRAS, PI3KCA, or B-Raf oncogenes, and their inhibition with targeted drugs has shown therapeutic immunomodulation effects. BRAF and MEK inhibitors, including FDA-approved drugs like vemurafenib, dabrafenib, and trametinib, mediate cancer cell-dependent immunostimulatory effects.
KRAS and Braf mutations in malignant cells support the establishment of an immunosuppressive microenvironment. BRAF and MEK inhibitors induce various cancer cell-dependent immunostimulatory effects, including upregulation of tumor-associated antigens, improvement of MHC I antigen presentation, induction of immunogenic cell death (ICD), secretion of TH1 cell cytokines like CXCL9 and CXCL10, and downregulation of immunosuppressive factors like IL8, VEGFA, and SPP1.
However, resistance to these inhibitors can lead to antigen presentation loss, TEFF cell exhaustion, and immunosuppressive cell infiltration. Combinations with other immunotherapies, such as CTLA-4 or TIM-3 blockers, immune-stimulating molecules, and CDK4/CDK6 inhibitors, may overcome these challenges.
DDR and Apoptosis-Targeted Drugs
Certain cancer cells heavily depend on DNA damage repair (DDR) mechanisms or robust anti-apoptotic signals, leading to the development of targeted anticancer drugs based on synthetic lethality principles. PARP1 inhibitors are licensed for cancers with DDR defects, while BCL2 inhibitors are approved for CLL or SLL patients. These DDR-targeted drugs exhibit therapeutic immunomodulatory effects.
PARP inhibitors, including FDA-approved and experimental ones, promote type I interferon secretion in various tumor cells. They enhance antigen presentation, activate dendritic cells (DCs), and upregulate MHC II molecules and co-stimulatory ligands to support T cell activation. PARP inhibitors also correlate with PD-L1 upregulation in cancer cells, potentially due to interferon signaling. Combinations with immune checkpoint inhibitors (ICIs) have been successful in preclinical tumor models.
ATM and ATR, two DDR-related kinases, have shown potent immunosuppressive effects. ATM cooperates with PARP1 to activate a non-classical STING-dependent program, leading to NF-kB-driven type I interferon and IL6 secretion. ATR inhibitors enhance CGAS signaling, type I interferon responses, and antigen presentation while favoring tumor infiltration by DCs and reprogramming TAMs towards immunostimulation, supporting T cell-dependent anticancer immunity.
BCL2, a DDR-driven anti-apoptotic protein, is a significant contributor to immune suppression. BCL2 inhibition can lead to effective antitumor immune responses, including downregulation of immunomodulatory cytokines, upregulation of NKALs, and induction of senescence-associated secretory phenotype (SASP) favoring recruitment and activation of NK cells, TEFF cells, and macrophages.
HER2, EGFR, VEGFA, and TGF-β Inhibitors
Over the last two decades, many monoclonal antibodies and tyrosine kinase inhibitors (TKIs) have been developed to target proteins or their binding partners, including HER2, EGFR, and VEGFA-specific drugs. While the safety and efficacy of TGF-β receptor or ligand inhibitors are still under investigation, these drugs have demonstrated therapeutic immunomodulatory effects.
HER2-targeted drugs like trastuzumab and pertuzumab, as well as EGFR inhibitors such as cetuximab and panitumumab, can improve antitumor immune responses through several mechanisms. These include antibody-dependent cellular cytotoxicity (ADCC), increased MHC I and II expression, and enhanced antigen presentation, leading to efficient T cell priming.
HER2 inhibitors, particularly in combination with immune checkpoint inhibitors (ICIs) like anti-PD-1 or anti-PD-L1, show enhanced antitumor effects. However, resistance mechanisms to these drugs often involve the activation of alternative survival pathways, escape from immune surveillance, and development of a highly immunosuppressive microenvironment. Combining HER2 inhibitors with immunomodulatory drugs or other targeted anticancer agents may overcome these challenges.
EGFR inhibitors can also trigger immune-mediated antitumor responses by promoting the maturation of DCs, increasing MHC I and II expression, and enhancing antigen presentation. Combining EGFR inhibitors with ICIs has been investigated in various cancers, and early clinical trial results suggest promising outcomes, although challenges related to acquired resistance, immune escape, and immunosuppressive microenvironments persist.
VEGFA inhibitors, including bevacizumab, ramucirumab, and axitinib, primarily target angiogenesis and have shown indirect immunomodulatory effects. These drugs can normalize tumor vasculature, increase T cell infiltration, and decrease immunosuppressive cell populations in the tumor microenvironment. The combination of VEGFA inhibitors with ICIs, adoptive cell therapy, or other immunomodulatory agents is a current focus of research.
TGF-β is a multifunctional cytokine with dual roles in tumorigenesis, acting both as a tumor suppressor and a promoter. The immunosuppressive functions of TGF-β make it a potential target for cancer therapy. Various TGF-β inhibitors, including ligand traps and receptor kinase inhibitors, are in preclinical or early clinical development. However, the complexity of TGF-β signaling, its context-dependent effects, and potential systemic toxicities pose challenges for targeting this pathway.
Conclusion
Targeted anticancer drugs have transformed cancer treatment paradigms by offering personalized and precise therapeutic approaches. While their primary focus is often on inhibiting specific molecular pathways in cancer cells, accumulating evidence suggests that many of these drugs possess significant immunomodulatory effects.
Understanding the immunomodulatory mechanisms of targeted anticancer drugs is crucial for maximizing their therapeutic potential. Harnessing these effects to improve the efficacy of immunotherapies, such as immune checkpoint inhibitors, adoptive cell therapies, or cancer vaccines, holds great promise for enhancing antitumor immune responses.
Combination strategies that exploit the immunomodulatory properties of targeted anticancer drugs can address the limitations of monotherapy, overcome resistance mechanisms, and create a synergistic effect on the immune system. This approach may lead to improved clinical outcomes, prolonged patient survival, and increased overall response rates across a wide range of cancer types.
As we delve deeper into the intricate interplay between targeted anticancer drugs and the immune system, ongoing research will likely uncover new immunomodulatory mechanisms, identify optimal combination strategies, and pave the way for the development of more effective and personalized cancer treatment regimens. The integration of immunomodulation with targeted therapies represents a promising frontier in the quest to unlock the full potential of the immune system in the fight against cancer.
Immunomodulatory Mechanisms of Targeted Anticancer Drugs
References:
1.Immunomodulation by targeted anticancer agents. Cancer Cell. 2020 Dec 17;S1535-6108(20)30601-2.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



