Diet choices determine the effect of cancer-targeted drugs?
- What are the WHO recommendations for Japanese encephalitis vaccines?
- Individuals Carrying Two APOE4 Copies Likely to Develop Alzheimer’s Disease Symptoms
- What Is The Role of Lactic Acid in Tumor Growth and Therapy Resistance?
- The Enigma of Beethoven’s Deafness: Unveiling the Role of Lead Poisoning
- World First Autologous Regenerated Islet Transplantation Successful
- FDA Approved Opdualag: The First Immunotherapy Targeting LAG-3
Diet choices determine the effect of cancer-targeted drugs?
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Diet choices determine the effect of cancer-targeted drugs? Progression-free survival doubled: 12.8 months vs 35 months.
Speaking of the second-generation ALK-targeted drug, everyone will definitely think of the famous alectinib. Before the launch of alectinib, it was compared with crizotinib head-to-head in the ALEX study with a median PFS of 34.8 months.
Surprised the entire oncology community.
The low water solubility of alectinib leads to poor absorption. The absolute bioavailability of alectinib after meals is only 36.9% (90% CI: 33.9%, 40.3%).
According to the data disclosed by the kinetics, the exposure after a single oral administration of 600 mg alectinib with a high-fat, high-calorie meal is 3 times higher than that of fasting medication, so it is recommended that alectinib be taken with meals.
The exposure of alectinib will further affect the therapeutic effect of alectinib (PFS). According to the pharmacokinetic report of alectinib disclosed by the FDA, the exposure of alectinib is less than 435 ng/mL and its effect on tumor Patients with tumor objective responses were lower than those with >435 ng/mL.
In a clinical research survey report, it was found that the trough blood concentration (Ctrough) of alectinib in 37% of patients did not reach the minimum exposure of 435 ng/mL, and the median PFS of these patients was 12.8 months.
Only 1/2 of normal patients .
Although alectinib needs to be taken with meals, different patients have different eating habits and dietary structures. Whether these dietary structures can affect the absorption of alectinib is unclear.
Alectinib is a fat-soluble drug, so foods with high fat content may enhance the solubility of Alectinib in the gastrointestinal tract and thus enhance the bioavailability of patients.
Recently, JNCCN, the official journal of NCCN in the United States, published a very interesting paper on whether different foods will affect the absorption of alectinib. Let’s see if this paper can verify the above point of view.
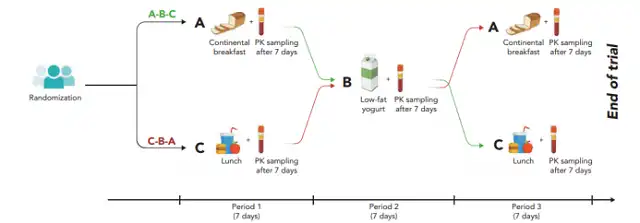
Figure: Study Design
This study is a single-center randomized crossover clinical study. 20 patients successfully enrolled were randomly divided into two groups designed as above: ABC group and CBA group.
Patients in the ABC group first received a European standard breakfast diet (A: two slices of buttered wheat bread + ham, cheese or peanut butter + 250 ml of semi-skimmed milk) + alectinib 7 days later to measure blood levels, and then received low-fat yogurt (B ) + alectinib 7 days later to measure the blood concentration, and finally the patient received the optional lunch (C) + alectinib 7 days later to measure the blood concentration;
the CBA group first received the optional lunch (C) + alectinib 7 days later Measuring blood drug concentration, measuring blood drug concentration after receiving low-fat yogurt (B) + alectinib for 7 days, and finally measuring blood drug concentration after receiving European standard diet (A) + alectinib for 7 days.
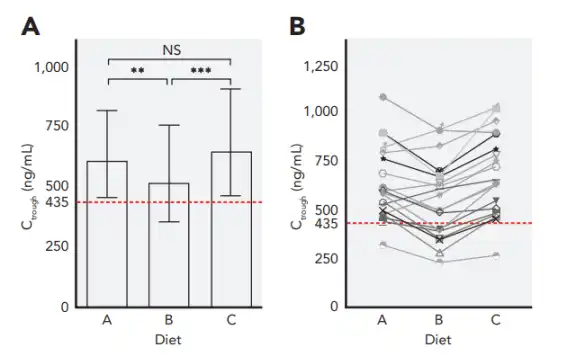
Figure: Effect of diet on the minimum plasma concentration of alectinib
The results of the study found that the average fat content of the standard European breakfast diet (A), low-fat yogurt group (B) and self-selected lunch group (C) was 21.3 g, 3.8 g and 19.5 g, respectively. The average trough blood concentration (Ctrough) levels of alectinib among the three were 607 ng/mL, 516 ng/mL and 645 ng/mL, respectively. Mean trough blood levels (Ctrough) in the low-fat yogurt group (B) were significantly lower than those in the European standard breakfast diet (A) by 14% (95% CI, -23 to -25; P = 0.009) and in the optional lunch group (C) 20% (95% CI, -25 to -14; P<0.001), there was no statistically significant change in the mean trough blood concentration (Ctrough) between the European standard breakfast diet (A) and the optional lunch group (C).
When 435 ng/mL was listed as the lowest threshold, it was found that there was only 1 patient in the European standard breakfast diet (A) and the optional lunch group (C) lower than 435 ng/mL, while 7 patients in the low-fat yogurt group (B) (35 %) was lower than 435 ng/mL (upper graph), there was a significant difference between the two (P = 0.002).
In terms of safety, the overall incidence of adverse reactions was low. There were 9 cases (45%) of adverse reactions in the European standard breakfast diet (A), 7 cases (35%) in the low-fat yogurt group (B) diet, and 7 cases (35%) in the optional lunch group ( C) There were 5 cases (25%) in the diet and there was no statistical difference among the three.
Most of the adverse reactions were grade 1, one grade 2 adverse reaction was reported in the low-fat yogurt group (B) and the self-selected lunch group (C), and there were no grade ≥ 3 adverse reactions.
Summary
This study verified that the dietary structure does affect the absorption of alectinib, and the exposure of alectinib in 35% of patients in the low-fat yogurt diet did not reach the standard.
10% of patients with adverse reactions of alectinib have adverse reactions of weight gain. Many physicians recommend that patients reduce the occurrence of such adverse reactions with a low-fat diet, but a low-fat diet is likely to increase the exposure of patients to alectinib.
Therefore, it is not recommended for patients to reduce fat intake in the diet with alectinib.
In addition, the author suggests that drug education for patients should be strengthened. The routine high-protein, low-fat healthy diet of cancer patients is not suitable for patients taking alectinib.
For alectinib, it must be taken with meals and the food must contain enough fat !
For patients with better economic conditions or patients with poor treatment effect of alectinib, it is recommended to perform blood drug concentration detection (TDM) of alectinib to improve the effectiveness and safety of alectinib medication.
references:
Lanser DAC, de Leeuw SP, Oomen-de Hoop E, de Bruijn P, Paats MS, Dumoulin DW, Koolen SLW, Dingemans AC, Mathijssen RHJ, Veerman GDM. Influence of Food With Different Fat Concentrations on Alectinib Exposure: A Randomized Crossover Pharmacokinetic Trial. J Natl Compr Canc Netw. 2023 Jun;21(6):645-651.e1. doi: 10.6004/jnccn.2023.7017. PMID: 37308124.
Diet choices determine the effect of cancer-targeted drugs?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.