Global Debut of Akalux: The First Light Immuno-Therapy ADC
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Global Debut of Akalux: The First Light Immuno-Therapy ADC
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Global Debut of Akalux: The First Light Immuno-Therapy ADC
Light Immuno-Therapy (PIT), pioneered by Julia Lev1wy at Columbia University in 1983, combines photodynamic therapy with immunotherapy. This approach boasts low toxicity, rapid efficacy, and does not develop drug resistance upon repeated use. It exhibits selectivity for tumor tissues, making it particularly suitable for treating metastatic tumors without harming normal tissues.
01. Akalux: The World’s First Light Immuno-Therapy ADC
Akalux (Cetuximab Sarotalocan Sodium, ASP-1929, RM-1929) is a light immuno-therapy ADC developed by Japan’s Rakuten Medical. In September 2020, it received official approval from the Japanese Ministry of Health, Labor, and Welfare for the treatment of unresectable head and neck malignancies. It stands as the world’s inaugural light immuno-therapy ADC, requiring conjunction with the BioBlade laser system during treatment.
Akalux, presented as a blue-green injectable solution with a pH of approximately 7.1, comes in 250 mg vials. Its components include anhydrous sodium hydrogen phosphate (42.6 mg), sodium dihydrogen phosphate monohydrate (27.6 mg), algal trehalose hydrate (4.5 g), and 10 mg of Tween 80.
Usage and Dosage:
Administered once daily at 640 mg/m2 (body surface area) via intravenous infusion for at least 2 hours. Laser irradiation of the lesion occurs 20-28 hours after infusion completion.
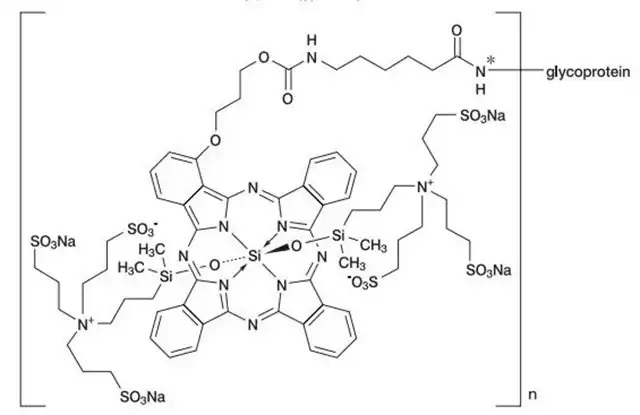
Akalux combines the anti-EGFR antibody cetuximab with the photosensitive substance IR700. Cetuximab, a chimeric anti-epidermal growth factor receptor (EGFR) monoclonal antibody (IgG1), is approved for colorectal and head and neck cancer treatment. Its payload is the photosensitive substance IR700, a water-soluble silicon phthalocyanine derivative sensitive to red visible light.
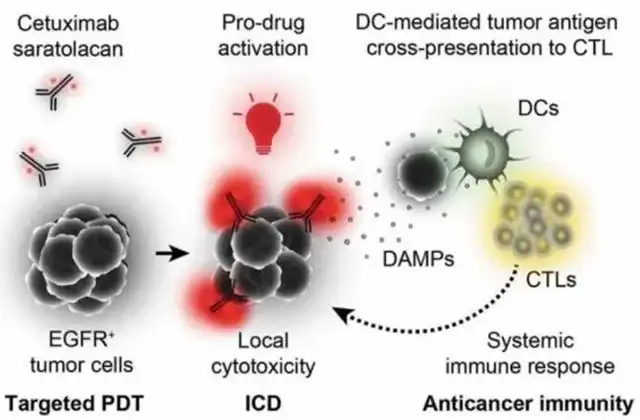
Mechanism of Action:
Cetuximab binds specifically to EGFR on tumor cell membranes. Subsequent near-infrared light exposure to the tumor site triggers the phototoxicity of IR700, precisely eliminating tumor cells while sparing surrounding normal tissue.
02. Clinical Studies
In September 2020, Akalux received approval in Japan. Based on international and Japanese Phase I/II trials, including Phase I/IIa trials on 30 recurrent head and neck cancer patients globally and Phase I trials on 3 Japanese patients, results indicate a response rate of 43.3% in the global Phase IIa trial and 66.7% in the Japanese Phase I trial.
Safety:
Common adverse reactions to Akalux include carotid artery bleeding, ulcer bleeding (5.6%), tongue swelling (13.9%), laryngeal swelling (5.6%), infusion reactions (2.8%), and severe skin disorders.
Globally, other light immuno-therapy ADCs in development include TROP-2-IR700, antibody C-IR700, ramucirumab-IR700, and MN-14-700DX, all in preclinical or drug discovery stages.
03. Comparative Analysis of 15 Global Marketed ADCs
Examining 15 approved ADCs globally, the time between the approval of the first and second ADC was the longest, spanning 11 years. Subsequent approvals saw a gradual reduction in intervals, with a peak of 9 new ADCs approved globally from 2019 to 2021. In 2022, only one new ADC was introduced, and no new products were approved in 2023.
All 15 ADCs are primarily indicated for tumors, with the initial ones mostly for non-solid tumors due to early technological limitations. China’s Aidixi® stands as the only ADC originating from China.
Antibody-wise, 13 ADCs use IgG1, while 2 utilize IgG4. Connectors vary, with 12 ADCs having cleavable connectors and 3 featuring non-cleavable connectors. In terms of toxic payload, 8 ADCs deploy microtubule-disrupting agents, 5 use DNA-damaging agents, and the remaining 2 utilize other toxic drugs.
Drug-to-antibody ratios (DAR) generally range between 2-4, a crucial parameter affecting ADC safety and efficacy. Commercially, three ADCs—Kadcyla, Adcetris, and Enhertu—have surpassed $1 billion in global sales by 2022.
Kadcyla, leading ADC sales in 2021 and 2022, faces potential displacement with Enhertu emerging as a strong contender. The narrative shifts in the ADC landscape, culminating in the emergence of China’s original ADC—Aidixi®.
Conclusion:
As we witness the rise of original Chinese pharmaceuticals, the global biopharmaceutical industry’s winter is gradually thawing.
While challenges persist, the era of Chinese innovation in pharmaceuticals has dawned, offering hope for a promising future in this dynamic field.
Global Debut of Akalux: The First Light Immuno-Therapy ADC
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



