Unraveling Iron Death: From Tumor Biology to Immunotherapy Synergy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Unraveling Iron Death: From Tumor Biology to Immunotherapy Synergy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Unraveling Iron Death: From Tumor Biology to Immunotherapy Synergy
Iron death is a recently discovered form of programmed cell death that plays a crucial role in tumor biology and treatment. This unique form of cell death, characterized by iron-dependent lipid peroxidation, is precisely regulated by cellular metabolism networks involving lipids, iron, and amino acids.
Different tumors exhibit varying sensitivities to iron death. Recent evidence suggests that triple-negative breast cancer (TNBC), a highly aggressive disease with limited treatment options, is particularly susceptible to inducers of iron death. This indicates that this novel form of non-apoptotic cell death serves as an attractive target for treating “hard-to-treat” tumors.
Interestingly, iron death has recently been linked to T cell-mediated anti-tumor immunity, influencing the efficacy of tumor immunotherapy. Therefore, a better understanding of this iron-dependent cell death could lead to the discovery of new cancer combination therapy strategies, holding significant biological and clinical implications.
Molecular Mechanism of Iron Death
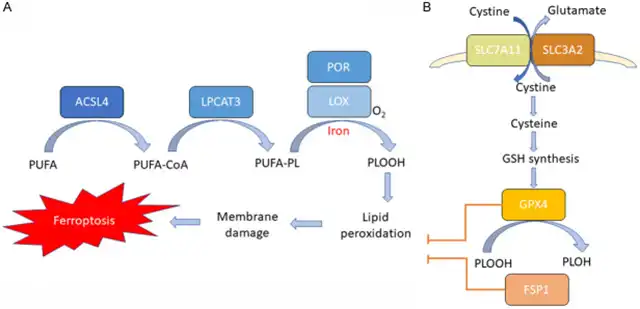
Iron death is a form of regulated cell death induced by iron-dependent lipid peroxidation. Three key features of iron death have been deciphered: membrane lipid peroxidation, intracellular iron availability, and loss of antioxidant defense.
Source: National Libary of Medicine
Lipid Peroxidation
Lipid peroxidation leads to the destruction of lipid bilayers and membrane damage, ultimately resulting in cell death. Cell membranes rich in phospholipids (PLs) containing polyunsaturated fatty acids (PUFAs) are highly susceptible to peroxidation induced by reactive oxygen species (ROS). The availability of membrane PUFAs capable of withstanding peroxidation is crucial for iron death.
Polyunsaturated fatty acids need to be synthesized, activated, and integrated into membrane PLs to participate in this death process, requiring two key enzymes, acyl-CoA synthetase long-chain family member 4 (ACSL4), and lysophosphatidylcholine acyltransferase 3 (LPCAT3). ACSL4 catalyzes the linkage of long-chain PUFAs with coenzyme A (CoA), while LPCAT3 facilitates the esterification of these products and their incorporation into membrane phospholipids.
Certain lipoxygenases (LOX) are considered primary enzymes capable of directly oxidizing membrane lipids containing PUFAs in the lipid bilayer. However, the mechanism by which LOX mediates iron death induction remains to be further studied. Another enzyme, cytochrome P450 oxidoreductase (POR), has recently been implicated in initiating lipid peroxidation.
Iron Accumulation
As the name “iron death” suggests, iron is essential for executing cell iron death. Iron is an indispensable element in the Fenton reaction, which generates free radicals and mediates lipid peroxidation. Additionally, iron is necessary for activating iron-containing enzymes such as LOX and POR, both of which are responsible for oxidizing membrane PUFAs. Furthermore, iron is crucial for the oxidative-reductive metabolism process involved in cellular reactive oxygen species production.
Given the critical role of iron in iron death occurrence, cellular iron pools are intricately controlled through the regulation of genes related to intracellular iron storage, release, input, and output. Fluctuations in cellular labile iron affect cell sensitivity to iron death. For example, increased iron input through transferrin or degradation of iron storage proteins can enhance cellular iron utilization and sensitize cells to iron death.
Loss of Antioxidant Capacity
Under normal conditions, iron-mediated lipid oxidation is tightly controlled by cellular antioxidant defense systems. Glutathione peroxidase 4 (GPX4) is considered a crucial antioxidant enzyme, acting directly to eliminate hydrogen peroxide in lipid bilayers and prevent the accumulation of lethal lipid ROS.
GPX4 utilizes glutathione (GSH) as a substrate to reduce membrane phospholipid hydroperoxides to harmless alcohols. The synthesis of GSH is essential for GPX4 activity and requires three amino acids: cysteine, glycine, and glutamate. Cysteine is a limiting substrate for glutathione synthesis and a vital component of glutathione synthesis. The abundance of intracellular cysteine in mammalian cells is primarily regulated by the xc system’s two subunits, SLC7A11 and SLC3A2. Small molecule inhibitors such as erastin, which inhibit cysteine uptake mediated by SLC7A11, can induce iron death in various cancers.
Recently, an alternative GPX4-independent iron death inhibition mechanism has been discovered. The ferroptosis suppressor protein 1 (FSP1)-CoQ system can protect cells from GPX4 inhibition-induced iron death. FSP1 prevents lipid peroxidation by reducing lipid radicals. Thus, cells utilize both the cysteine-GSH-GPX4 and FSP1-CoQ axes to inhibit lipid peroxidation and prevent iron death. When these antioxidant defense systems are overwhelmed by iron-dependent lipid ROS accumulation, iron death occurs.
Iron Death in TNBC
Different types of cancers exhibit varying sensitivities to iron death. Recent evidence suggests that alterations in iron death-related metabolic pathways (such as lipid, iron, and amino acid metabolism) in TNBC make this refractory tumor inherently susceptible to iron death. The particular sensitivity of TNBC to iron death highlights the attractiveness of this non-apoptotic death pathway as a target for TNBC therapy.
Lipid Metabolism
Dysregulated lipid metabolism can lead to lipid peroxidation and iron death, with ACSL4 being a critical component of iron death execution. Interestingly, studies have found that compared to other types of breast cancer, ACSL4 is preferentially expressed in TNBC, and its expression predicts sensitivity to iron death. A recent study also observed significant upregulation of ACSL4 in TNBC tumors and cell lines. Given that ACSL4 enriches cell membranes with long-chain PUFAs, indicating TNBC’s abundance of PUFAs, it is particularly sensitive to iron death.
Iron Metabolism
Adequate intracellular iron is a prerequisite for executing iron death. Compared to normal cells, cancer cells exhibit higher dependency on iron to promote growth. A recent study indicated that genes regulating intracellular iron levels are significantly upregulated in TNBC compared to non-TNBC tumors and cell lines. Particularly, abundant low levels of iron export transporters were observed in TNBC, accompanied by high expression of iron import transferrin receptor. These alterations in gene expression involved in iron metabolism regulation may contribute to increased cellular labile iron pools, promoting iron-dependent lipid peroxidation, making TNBC a tumor rich in iron prone to iron death.
Amino Acid Metabolism
Amino acid metabolism is crucial for the antioxidant defense system composed of SLC7A11-mediated cystine uptake, GSH biosynthesis, and GPX4 activity. Cancer cells may exhibit dependency changes in specific amino acid metabolic pathways. An early study found that compared to other types of breast cancer, TNBC shows significant dependence on glutamine metabolism required for supplementing SLC7A11, suggesting a potential connection between TNBC and iron death.
Furthermore, compared to non-TNBC tumors, TNBC tumors exhibit reduced expression of glutathione synthetase (GSS), a key enzyme in GSH synthesis. Expression of GPX4 is also lower in TNBC compared to other types of breast cancer. Reduced intracellular GSH and GPX4 expression may weaken antioxidant defense capabilities, increase the likelihood of lipid peroxidation, and render TNBC particularly sensitive to agents promoting iron death.
Iron Death in Tumor Immunotherapy
Recent findings suggest that iron death contributes to the anti-tumor effects of CD8+ T cells and influences the efficacy of anti-PD-1/PD-L1 immunotherapy. Combining immunotherapy with modalities that promote iron death, such as radiotherapy and targeted therapy, can synergize to promote tumor control.
Combination of Immunotherapy with Cysteine Restriction
Recent reports suggest that CD8+ T cells activated by anti-PD-L1 immunotherapy secrete IFN-γ after PD-L1 blockade, promoting tumor cell iron death. Secreted IFN-γ significantly downregulates the expression of SLC3A2 and SLC7A11 in tumor cells, leading to reduced cystine uptake, enhanced lipid peroxidation, and subsequent iron death. The synergistic action of cysteine/cysteamine with anti-PD-L1 induces effective anti-tumor immunity by inducing iron death.
Combination of Immunotherapy with Targeted Therapy
A recent study indicates that resistance to anti-PD-L1 therapy can be overcome by combining it with receptor tyrosine kinase (RTK) inhibitors such as TYR03, which promote iron death. Increased expression of TYR03 has been observed in anti-PD-1-resistant tumors. Mechanistically, upregulation of key iron death genes such as SLC3A2 in the TYR03 signaling pathway inhibits tumor iron death. In TNBC mouse models with the same gene, inhibiting TYR03 promotes iron death and sensitizes tumors to anti-PD-1 therapy. This study reveals that relieving iron death by using TYR03 inhibitors is an effective strategy to overcome immunotherapy resistance.
Combination of Immunotherapy with Radiotherapy
Recent evidence suggests that the synergistic effect of radiotherapy with immunotherapy is associated with increased sensitivity to iron death. Radiation has been shown to induce iron death, and genetic and biochemical characteristics of iron death have been observed in cancer cells treated with radiation.
The mechanism involves radiation-induced ROS generation and upregulation of ACSL4, leading to enhanced lipid synthesis, increased lipid peroxidation, and subsequent membrane damage.
Thus, the anti-tumor effect of radiotherapy can be attributed not only to DNA damage-induced cell death but also to induced iron death.
Radiotherapy in combination with immunotherapy downregulates SLC7A11, mediated by DNA damage-activated kinase ATM and IFN-γ, resulting in reduced cystine uptake, increased iron death, and enhanced tumor control.
These studies reveal iron death as a new mechanism for the synergistic action of immunotherapy and radiotherapy.
Combination of Immunotherapy with T Cell Iron Death Inhibitors
In addition to inducing tumor iron death, T cells themselves may undergo iron death, which could weaken their immune response. GPX4-deficient T cells rapidly accumulate membrane lipid peroxides, leading to iron death. Similar to cancer cells, ACSL4 is essential for CD8+ T cell iron death and their immune function.
Recently, two studies have shown increased expression of CD36 in CD8+ tumor-infiltrating lymphocytes (TILs). Inherent CD36 in T cells promotes uptake of oxidized lipids and induces lipid peroxidation, resulting in impaired CD8+ T cell function. These findings reveal CD8+ T cell iron death as a new mode of tumor immune suppression and emphasize the therapeutic potential of blocking CD36 to enhance anti-tumor immunity. It is worth noting that the study also demonstrates the role of GPX4 in regulating the anti-tumor function of CD8+ TILs. Therefore, therapeutic induction of iron death in cancer cells by GPX4 inhibitors may produce unnecessary off-target effects on T cells and result in adverse toxicity.
Conclusion: Unraveling Iron Death: From Tumor Biology to Immunotherapy Synergy
Iron death is driven by oxidative lipid containing PUFAs, intracellular iron accumulation, and loss of antioxidant defense. Regulation of iron death plays a role in various cancer types, including TNBC.
Particularly, TNBC exhibits a unique pattern of expression of iron death-related genes, making it particularly susceptible to iron death. Therefore, targeting iron death for this refractory tumor may be a promising therapeutic strategy.
Furthermore, iron death plays a significant role in T cell-mediated anti-tumor immunity, influencing the efficacy of immunotherapy.
Combining immunotherapy with modalities that directly or indirectly induce iron death, such as radiotherapy and targeted therapy, offers a promising combination to improve anti-PD-1/PD-L1 immunotherapy.
Therefore, it is necessary to further explore ways to modulate iron death in combination with immunotherapy.
These findings will broaden and deepen our understanding of this novel form of cell death and provide new opportunities for future research directions.
Unraveling Iron Death: From Tumor Biology to Immunotherapy Synergy
References:
1.Ferroptosis: a promising target for cancer immunotherapy. Am J Cancer Res. 2021; 11(12): 5856–5863.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



