FDA preliminary analysis suggests RSV vaccine may increase risk of severe autoimmune disease
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA preliminary analysis suggests RSV vaccine may increase risk of severe autoimmune disease
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA preliminary analysis suggests RSV vaccine may increase risk of severe autoimmune disease
Recent data from the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) in the United States suggest that a new vaccine designed to protect older adults from respiratory syncytial virus (RSV) may be associated with an increased risk of developing Guillain-Barre syndrome (GBS).
GBS is a typical post-infection autoimmune disease, often characterized by a rapid, monophasic course that typically does not recur.
About two-thirds of adult patients report prodromal symptoms of respiratory or gastrointestinal infection in the four weeks before the onset of weakness; 20-30% of cases will progress to severe systemic illness with respiratory failure.
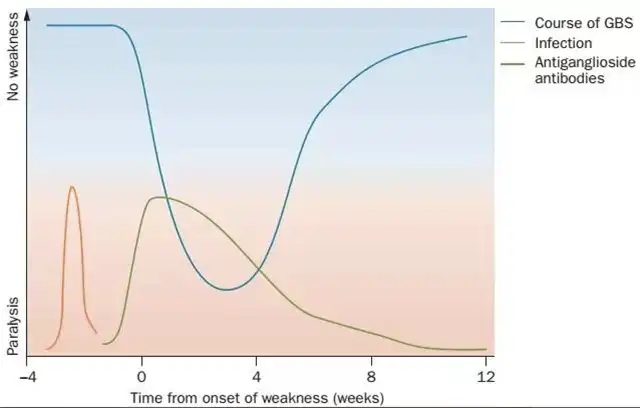
Figure 1: Curve chart of the course of GBS . The blue curve is the progression of GBS. Progressive limb weakness reaches a peak within 4 weeks (often within 2 weeks), and the recovery period can last for weeks, months or even years. The red curve represents that most GBS patients often have pre-infections before the onset of limb weakness; the green curve represents that anti-ganglioside antibodies are often detectable, but their levels decrease over time.
The severity and duration of GBS vary greatly. It can range from mild weakness that spontaneously resolves to limb paralysis and respiratory dependence, with no signs of recovery for months or even years. However, all patients eventually begin to improve, although their recovery may be prolonged, leading to severe permanent disabilities. During the acute, stable, or recovery phase of the disease, patients may experience symptoms or signs of autonomic dysfunction, such as occasional heart rhythm abnormalities requiring pacemaker, sweating, unstable blood pressure, or intestinal obstruction.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Thursday, vaccine safety experts from both agencies presented data indicating an apparent increase in the incidence of GBS among those vaccinated with the Pfizer vaccine, although cases were also found among those vaccinated with GSK products.
However, it is still too early to determine if there is an increased risk of developing GBS after receiving the RSV vaccine or to quantify the size of this risk, if any.
Tom Shimabukuro, former director of the CDC’s Immunization Safety Office and current deputy director of the Influenza Division, submitted GBS data to the ACIP meeting, stating that some of the data and findings were based on a small number of cases and relatively low doses. Due to uncertainty and limitations, based on these early data, it is unclear whether the risk of developing GBS after vaccination with the RSV vaccine is increased in those aged 60 and older. The CDC will begin more in-depth analysis using different vaccine safety databases in March, and better risk assessment results are expected in the coming weeks and months.
Some vaccines appear to increase the risk of GBS in recipients. However, determining whether there is a true link between vaccines and GBS can be tricky. Because in some cases, even if the risk of developing GBS increases after vaccination, this information must be evaluated in a broader context. For example, although the risk of developing GBS after receiving the influenza vaccine is believed to be slightly increased — with one or two more cases per 1 million people vaccinated compared to what is usually seen in a similar-sized unvaccinated population — it is well known that influenza infection also increases the risk of GBS.
Concerns arose as three cases of GBS were identified in the vaccine group in the clinical trials of RSV vaccines conducted by Pfizer and GSK.
As of mid-February, CDC analysts identified and verified 23 cases of GBS among approximately 9.5 million people who received one of these two vaccines. Of these, 15 received the Pfizer RSV vaccine (Abrysvo) and 8 received the GSK RSV vaccine (Arexvy). Complicating the issue is that 14 of the cases involved simultaneous administration with another vaccine, including various brands of influenza and Covid-19 vaccines, as well as herpes zoster, tetanus-diphtheria-pertussis, and rabies vaccines.
Within 21 days of vaccination, the GBS incidence rate for recipients of the Pfizer Abrysvo vaccine was 4.6 cases per 1 million doses, while for the GSK Arexvy vaccine, this rate was 1.1 cases per 1 million doses. Shimabukuro noted that the expected background rate — the rate of GBS occurring in a vaccinated population where vaccination does not increase the risk of GBS — is 2.0 cases per 1 million doses within 21 days of vaccination.
Thus, based on the current data set, the Pfizer RSV vaccine has achieved statistical significance in increasing the risk of GBS, but there is no evidence to suggest that the GSK RSV vaccine increases the risk of GBS.
In response, Pfizer and GSK stated:
Reema Mehta, Vice President and Head of Global Safety Risk Assessment and Management at Pfizer, told ACIP that the company believes its vaccine is safe but is conducting four post-market safety studies to look for GBS in recipients. The company will continue to share its findings with the CDC, FDA, and other stakeholders.
Alison Hunt, a spokesperson for GSK, pointed out in an email that the CDC’s analysis did not show an increased incidence of GBS in people vaccinated with the company’s RSV vaccine. However, the company has committed to initiating a controlled epidemiological study to evaluate the risk of GBS, and is currently developing the protocol for this study.
CDC vaccine experts stated that their risk-benefit analysis continues to support the use of the RSV vaccine in people aged 60 and older, as this population bears a significant burden of RSV disease. The agency estimates that for every 1 million doses of RSV vaccine administered, 2400-2700 RSV hospitalizations, 450-520 intensive care unit admissions, and 120-140 deaths can be avoided.
As this signal emerges, GSK hopes to persuade the FDA to expand the licensure of its RSV vaccine to lower the age of eligible recipients, including those aged 50-59, who are at risk of severe illness from RSV infection.
Meanwhile, the RSV Working Group of the ACIP (a subcommittee of the committee composed of ACIP members, CDC staff, and external experts) is considering whether the CDC’s recommendations for who should receive the RSV vaccine should change. The current policy approved last fall recommends vaccination for people aged 60 and older, provided healthcare providers deem it beneficial. Survey data suggest that this “shared decision-making” recommendation has confused both physicians and some potential recipients and may have suppressed vaccination rates.
It is worth noting that there have been precedents for other vaccines being withdrawn from the market due to causing rare diseases: the first rotavirus vaccine was withdrawn from the market in 1998 due to rare cases of intussusception. If the increased risk of GBS due to the RSV vaccine is confirmed and the regulatory agencies assess that the risk outweighs the benefit, it could also lead to the withdrawal of market approval.
Do you think the RSV vaccine will become history?


FDA preliminary analysis suggests RSV vaccine may increase risk of severe autoimmune disease
References:
[1]RSV vaccines may be linked to small increased risk of developing Guillain-Barré syndrome, data suggest.STAT.By Helen Branswell. Feb. 29, 2024.
[2]RSV vaccines may raise risk of Guillain-Barré syndrome, FDA preliminary analysis finds.By Zoey Becker.Mar 4, 2024 11:46am.
[3] Detailed explanation of neurological diseases: Guillain-Barré syndrome. iNeurology Navigation. 2022-12-19.
[4] The classification, manifestations, and treatment of Guillain-Barré syndrome…this article explains it clearly! .Lilac Garden Neural Time.2024-01-19.
[5] RSV vaccine may increase the risk of rare neurological diseases! .Da Yao Shi Ji.2024-03-01.
[6] “Preliminary Analysis of Guillain-Barré Syndrome (GBS) following RSV Vaccination among adults 65 years and older”.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



