FDA Delays Approval of Eli Lilly’s Donanemab for Alzheimer’s disease
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Breaking News! FDA Delays Approval of Eli Lilly’s Donanemab for Alzheimer’s disease
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA Delays Approval of Eli Lilly’s Donanemab for Alzheimer’s disease, Causing Speculation…
Eli Lilly announced on March 8, 2024, that the FDA plans to convene the Peripheral and Central Nervous System Drugs Advisory Committee (PCNSDAC) to discuss the Phase III clinical trial of their Alzheimer’s disease drug, Donanemab, known as the TRAILBLAZER-ALZ 2 study, to evaluate its efficacy and safety in early symptomatic Alzheimer’s disease (AD).
Due to the uncertainty of the PCNSDAC meeting date, the FDA has decided to further postpone the Prescription Drug User Fee Act (PDUFA) date for Donanemab’s approval.

Since the accelerated approval of Aduhelm, developed by Eisai/Biogen, on January 6, 2023, breaking a 20-year silence in the AD field (excluding the controversial Aduhelm), there has been great anticipation in the market for Donanemab to be compared with it.
However, unlike the smooth approval of Lecanemab, the approval process for Donanemab has been tumultuous.
On January 19, 2023, Donanemab, which was intended to be approved through the fast-track policy, was rejected by the FDA due to insufficient clinical trial sample size (only 94 subjects in the trial group completed the trial).
Forced to do so, Eli Lilly had to rely on the large-scale Phase III clinical trial TRAILBLAZER-ALZ 2 as evidence, seeking the traditional approval pathway, and submitted the marketing application to the FDA in Q2 of the same year.
According to Eli Lilly’s historical official announcements, the original PDUFA date for this marketing application should have been the end of 2023, but it was later postponed to Q1 of 2024. Now, with Q1 almost over and the approval plan delayed again, an expert advisory committee has also been thrown into the mix.
As a benchmark for global drug regulatory agencies, the FDA’s approval decision may directly affect the registration progress in other regions. The FDA’s indecisive attitude is indeed thought-provoking.
Same Origin, Different Fate
Due to the complex pathogenesis of AD, there are multiple viewpoints on the pathogenic hypothesis in the academic community, with the major one being the “Amyloid-β (Aβ) hypothesis,” which states that the deposition of Aβ forms amyloid plaques, driving neurodegenerative processes leading to disease.
Donanemab and Lecanemab are the two most prominent drugs developed based on the Aβ hypothesis. However, although these two drugs are from the same origin, their mechanisms are different. Understanding the differences between these two drugs may further speculate on the potential reasons for the FDA’s hesitancy.
The full name of the “Aβ hypothesis” should be the “Aβ Cascade Hypothesis,” which describes the dynamic process of Aβ pathogenicity.
Aβ is a cleavage product of amyloid precursor protein (APP). APP is a common protein in the human body, mainly concentrated in the synapses of neurons. Under normal circumstances, APP is cleaved into two fragments, which are not only harmless but also helpful in maintaining normal brain function. However, when APP is cleaved incorrectly, it produces harmful Aβ.
It’s like a frying pan; when separated normally, there’s a lid on top and a pan below for cooking. But when separated incorrectly, it becomes a pan handle, a lid, and a “pancake.” At this point, the lid can still cover the remaining food, and the “pancake” can still be sold as iron, but the handle can only be treated as garbage.
This handle is Aβ.
Of course, the immune system can clear Aβ. It’s only when Aβ is produced in large quantities and there are problems with the immune system that the cascade reaction occurs. At this point, Aβ continues to aggregate, forming oligomers; oligomers further aggregate, forming protofibrils; protofibrils then aggregate, forming insoluble plaques.
At this point, there are divergent views in drug development.
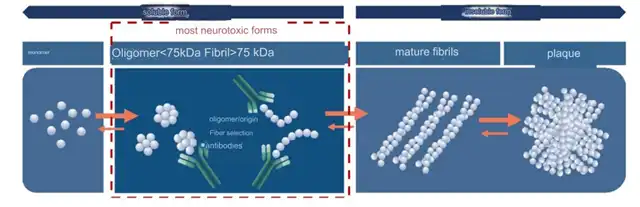
The developers of Lecanemab believe that the soluble oligomers and protofibrils formed during Aβ aggregation have direct neurotoxicity, which can induce synaptic dysfunction. After the formation of plaques, the toxicity decreases. Therefore, the focus should be on protofibrils, which reduces toxicity and stops the continued deposition of plaques, thereby preventing the progression of AD.
On the other hand, the developers of Donanemab believe that merely preventing the deposition and growth of new plaques cannot solve the fundamental problem. The focus should still be on existing plaques in the brain, which have already caused serious burdens on the brain and should be removed decisively.
However, there is a risk in “clearing plaques.” These plaques are too large and entangled with neurons. Directly targeting them could affect many normal tissues, leading to brain hemorrhage and edema.
Although Lilly has claimed that Donanemab is different from previous “plaque-clearing” drugs, early studies have confirmed that Donanemab does not cause brain hemorrhage in animal experiments. However, this situation is still unavoidable in large-scale human trials.
Comparing the safety results of Clarity AD and TRAILBLAZER-ALZ 2, it can be seen that the risk of ARIA-E (cerebral edema) and ARIA-H (cerebral hemorrhage) in Donanemab patients is much higher than that in Lecanemab patients. Moreover, there were 3 deaths considered treatment-related in the Donanemab group.
This may be one of the motivations for the FDA to convene an expert advisory committee for discussion.
Questioning Effectiveness
The announcement from Lilly stated that the FDA wants to understand two things:
- The safety of patients treated with Donanemab;
- Whether the unique trial design of TRAILBLAZER-ALZ 2 will interfere with the evaluation of drug effectiveness.
The first point is easy to understand, as it can be seen from the data on adverse events in the study. The second point is interesting – how unique is the trial design really?
In the complete data of the TRAILBLAZER-ALZ 2 study published in JAMA, there is a subsection titled “Randomization and Intervention,” which explains:
The trial duration is 72 weeks, with PET scans at weeks 24 and 52 to assess the subjects’ amyloid plaque levels. If the Centiloid level is less than 11 in any single scan or greater than 11 but less than 25 in two consecutive PET scans, Donanemab will be switched to placebo in a blinded procedure.
Let’s break it down.
Centiloid is a quantification unit for amyloid PET imaging. The Centiloid status of healthy young people is defined as 0, and the Centiloid status of clearly AD patients is defined as 100. The rest of the operation is similar to Celsius temperature; average it out to achieve comparability between different imaging results.
If the test result is less than 25 Centiloids, it is considered amyloid-negative, similar to healthy young people.
Since the inclusion criterion for patients is ≥37 Centiloids, we can understand that patients who meet the above criteria have
achieved pathological complete remission. At this point, without informing the patient, Donanemab is secretly switched to placebo (Donanemab is switched to placebo in a blinded procedure).
The FDA believes that this dosing regimen based on the evaluation of patient amyloid levels may affect the evaluation of Donanemab’s effectiveness.
In my personal opinion, this evaluation does have flaws.
Firstly, it is questionable whether Centiloid levels and disease status are equivalent; otherwise, why would you choose the iADRS cognitive scale as the primary endpoint?
Secondly, how are these patients treated afterward? Are they always on placebo, or if they progress after stopping treatment, do they revert to Donanemab therapy? Are these patients who did not complete the full 76 weeks of treatment included in the final overall assessment, and is this favorable or unfavorable for positive results?
Next door, Lecanemab in the Clarity AD study did not have this operation, after all, it is not for “clearing plaques,” so the statistical results are easier to understand.
In addition to treatment duration, the inclusion criteria for the TRAILBLAZER-ALZ 2 trial are significantly different from those of Clarity AD. One critical point is that TRAILBLAZER-ALZ 2 requires patients to have pathological features of Tau protein and divides patients into “low/medium Tau group” and “full population” for analysis based on Tau protein levels in the primary endpoint.
This approach actually excludes “low Tau patients” from the study, and “low Tau” is actually a feature of early AD. Will this hinder the early use of Donanemab? After all, you are trying to prove the efficacy of Donanemab in early treatment of AD, but you are excluding AD patients with “early low tau.”
This is also one of the topics that the FDA wants to discuss, which may only be revealed at the expert advisory committee meeting.
In conclusion, the twists and turns of Donanemab’s approval have once again elevated the historical status of Lecanemab. As the first AD treatment drug to receive full FDA approval in 20 years, the success of Lecanemab can be said to be built on the “corpses” of its predecessors.
The American Association of Pharmaceutical Manufacturers once published a report indicating that between 1998 and 2017, there were 146 Alzheimer’s disease drugs that failed in clinical trials globally, with only four drugs targeting disease symptoms approved for marketing. This means that the clinical success rate of new AD drugs is only 2.7%.
We look forward to Donanemab passing the FDA’s test, proving its clinical value with strong evidence, benefiting more AD patients, and inspiring more researchers dedicated to conquering AD.
Also, we look forward to the day when Alzheimer’s disease is finally ended by humanity.
Breaking News! FDA Delays Approval of Eli Lilly’s Donanemab for Alzheimer’s disease
References:
[1] Eli Lilly official website
[2] Eisai official website
[3] Demattos RB, Lu J, Tang Y, Racke MM, Delong CA, Tzaferis JA, Hole JT, Forster BM, McDonnell PC, Liu F, Kinley RD, Jordan WH, Hutton ML. A plaque-specific antibody clears existing β -amyloid plaques in Alzheimer’s disease mice. Neuron. 2012 Dec 6;76(5):908-20. doi: 10.1016/j.neuron.2012.10.029. PMID: 23217740.
[4] Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, Wessels AM, Shcherbinin S, Wang H, Monkul Nery ES, Collins EC, Solomon P, Salloway S, Apostolova LG, Hansson O, Ritchie C, Brooks DA, Mintun M, Skovronsky DM; TRAILBLAZER-ALZ 2 Investigators. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023 Aug 8;330(6):512-527. doi: 10.1001/jama.2023.13239. PMID: 37459141; PMCID: PMC10352931.
[5] KlunkWE, KoeppeRA, PriceJC, et al. The Centiloid project: standardizing quantitative amyloid plaque estimation by PET[J]. Alzheimers Dement, 2015, 11(1):1-15.e1-e4. DOI: 10.1016/j .jalz.2014.07.003.
[6] van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023 Jan 5;388(1):9-21. doi: 10.1056/NEJMoa2212948. Epub 2022 Nov 29. PMID: 36449413.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



