V-A ECMO support treatment on the microcirculation of patients
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
V-A ECMO support treatment on the microcirculation of patients
V-A ECMO support treatment on the microcirculation of patients. It is well known that both sepsis and cardiogenic shock suffer from microcirculation dysfunction, and microcirculation dysfunction is closely related to the prognosis of the disease.
Background:
Both sepsis and cardiogenic shock suffer from microcirculation dysfunction, and microcirculation dysfunction is closely related to the prognosis of sepsis. In patients with cardiogenic shock receiving VA-ECMO supportive treatment, there are few studies on microcirculation dysfunction and its prognosis.
Methods:
Use event dark field imaging technology to record sublingual microcirculation images at 12 hours (T1), 24 hours (T2), 48 hours (T3), 72 hours (T4) and 96 hours (T5) after VA-ECMO assistance . If the patient can be weaned off, record the sublingual microcirculation images before and after VA-ECMO is stopped. The sublingual microcirculation parameters of the dead and surviving patients who received VA-ECMO supportive treatment within 28 days were compared. In addition, microcirculation images and clinical data were used as a prognostic assessment of 28-day mortality, and patients were divided into three subgroups based on microcirculation parameters for survival analysis.
Results:
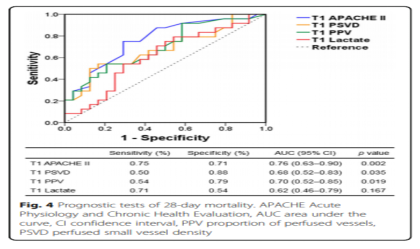
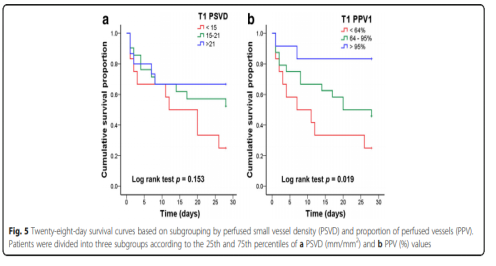
A total of 48 patients were included in this study. At T1, there was no significant difference in heart rate, mean arterial pressure, muscle strength score, and lactate level between the dead and surviving patients, but the perfusion vessel density (PSVD) and the perfusion vessel ratio (PPV) of the dead patients were lower than those of the survivors. PSVD and PPV are slightly better than lactate levels in predicting 28-day mortality (area under the curve 0.68, 0.70, and 0.62, respectively). Compared with the other two subgroups, the subgroup with the lowest PSVD (<15 mm/mm2) and PPV (<64%) values had a lower survival rate.
Conclusion:
Early microcirculation parameters can be used to predict the prognosis of patients with cardiogenic shock assisted by VA-ECMO.
Keywords: cardiogenic shock, extracorporeal membrane oxygenation, microcirculation, prognosis
Background:
ECMO extracorporeal life support provides cardiac and respiratory support for patients with heart failure, respiratory failure, or both. It provides time for the recovery of failed organs or further treatment. However, despite the support of ECMO, many patients died.
One of the key factors is whether ECMO assisted support can provide adequate perfusion for each organ. Good microcirculation may depend on arterial blood pressure and auxiliary flow of VA-ECMO. However, whether the microcirculation can be restored is still the main unresolved clinical problem of ECMO patients. Microcirculation dysfunction has been observed in patients with sepsis or cardiogenic shock and those who have undergone surgery. In addition, microcirculation dysfunction in patients with severe sepsis and out-of-hospital cardiac arrest is associated with a poor prognosis.
However, the relationship between VA-ECMO support patients’ microcirculation dysfunction and its prognosis is unclear. Therefore, this study used the third-generation video microscope to observe the microcirculation within 12 hours after VA-ECMO assistance, and compared the PSVD between the 28-day dead patients and the surviving patients. In addition, microcirculation and clinical parameter evaluation were used as prognostic indicators for 28-day mortality, and patients were divided into three subgroups based on microcirculation parameters for survival analysis.
Methods:
Patient information
This study was approved by the Research Ethics Committee of National Taiwan University Hospital (approval number: 201412045RINA) and registered on the ClinicalTrials.gov registration system. The study was conducted at National Taiwan University Hospital from June 2015 to August 2016. All patients received ECMO supportive treatment. ECMO was screened and evaluated within 12 hours after placement.
Inclusion criteria: patients with cardiogenic shock and receiving VA-ECMO supportive treatment. Exclusion criteria: patients under the age of 20 or over 80; patients who cannot measure the sublingual microcirculation within 12 hours after VA-ECMO assistance; patients who cannot communicate with each other.
The informed consent of the patient and his guardian must be obtained before participating in the study. An event dark field microscope (CytoCam, Braedius Medical, Huizen, Netherlands) was used to record sublingual microcirculation images. Images were recorded at the following time points after VA-ECMO assistance: 12 hours (T1), 24 hours (T2), 48 hours (T3), 72 hours (T4) and 96 hours (T5). If the patient can be weaned, record the sublingual microcirculation images at the following time points: before weaning (R0), 6 h (R1), 24 h (R2), 48 h (R3) and 72h (R4) after weaning .
VA-ECMO equipment and intubation
All included patients received VA-ECMO in the femoral artery and vein. The main equipment of VA-ECMO includes heparin pre-filling pipeline, centrifugal pump (BPX-80 Bio-Pump Plus, Medtronic), oxygenator (Affinity NT, Medtronic), air-oxygen mixer (3500 CP-G gas mixer) , Sechr-ist, Anaheim, USA) and intubation (BE-HLS, Maque, Turkey). In order to avoid possible underperfusion of the distal limb, when the average pressure of the superficial femoral artery is lower than 50 mmHg, the lower limb arterial perfusion tube should be used for distal limb perfusion. All patients received standard care management in VA-ECMO and intensive care unit (ICU).
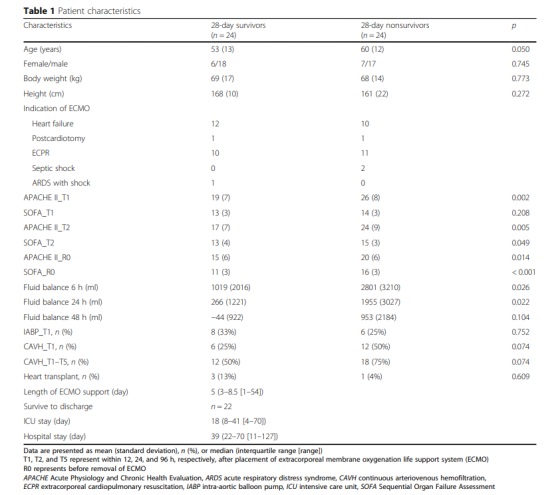
Record the following parameters: age, sex, height, weight, APACHE II score, SOFA score, VA-ECMO auxiliary indication, VA-ECMO auxiliary flow, heart rate, mean arterial pressure (MAP), lactate level, activated clotting time, hemoglobin, Body fluid balance and vasoactive drug score. Vasoactive drug score = 100×adrenaline dose (ug/kg/min) + 100×norepinephrine dose (ug/kg/min) + dopamine dose (ug/kg/min) + dobutamine dose ( ug kg / min). If aortic balloon pump or continuous arteriovenous hemofiltration is used at the same time with the support of VA-ECMO, it should be recorded at the same time.
The time of VA-ECMO assistance, the time of transfer to ICU, the length of hospitalization and 28-day survival status were also recorded. If active bleeding or other complications are not observed, continuous infusion of heparin should maintain the activated clotting time (ACT) at 160-180 s.
Measurement of sublingual microcirculation
At each point in time, five videos (with a duration of 6 s) from different sublingual parts were recorded and stored after being named after numbers to ensure that patient information was not leaked. The analysis was carried out by an observer who had no knowledge of the patient’s information. The statistical software AVA 3.0 (University of Amsterdam Medical Center, Amsterdam, Netherlands) was used for analysis. According to the microcirculation assessment consensus, monitor the following parameters: a) total small vessel density (less than 20μm) (TSVD); b) perfusion vessel density (PSVD); c) perfusion vessel proportion (PPV); d) microvascular blood flow index (MFI) ); e) Heterogeneity Index (HI). TSVD is automatically calculated by the software. According to the method described in the previous study, the numbers 0-3 are used for semi-quantitative classification of blood flow in small vessels. Small blood vessels with a blood flow classification of 2 or 3 are considered as perfusion vessels, and the PSVD is automatically calculated by statistical software. According to the consensus of microcirculation assessment, the MFI score and HI were calculated semi-quantitatively. The main purpose is to determine the difference in PSVD at T1 between the 28-day survivor and the dead. According to our experience, assuming that the average PSVD of the control group is 20 mm/mm3, the standard deviation is 4, α is 0.05, and β is 0.2, then 20 patients in each group detect a difference of 17.5% in PSVD between the two groups.
Prognostic evaluation and subgroup survival analysis of 28-day mortality
The ROC curve and the area under the curve were used to analyze the APACHE II score, lactate level, PSVD and PPV at T1 time to evaluate the 28-day mortality of the patient. Calculate the cut-off point of the ROC curve by obtaining the best Uden index (sensitivity + specificity-1). In addition, according to the 25th and 75th percentiles of PSVD and PPV, patients were divided into three groups, and the 28-day survival rate between the three groups was analyzed.
Statistical Analysis
Use SPSS 20 statistical software for data analysis. The measurement data according to the normal distribution is expressed as the mean (standard deviation), and the data of the 28-day survival patients and the dead patients are compared using the t test. Non-normally distributed measurement data and MFI scores are expressed as medians (quartiles), and the data of 28-day surviving patients and those who have died were compared using the Mann-Whitney test. Categorical variables are expressed as percentages and compared using Chi-square test or Fisher’s exact test as needed. P value<0.05 is considered as statistically significant.
Results
Patient characteristics
A total of 246 patients receiving VA-ECMO support were screened and determined whether they were eligible to participate in the trial. A total of 45 patients who received V-V ECMO did not meet the inclusion criteria, and another 153 patients were excluded (Figure 1). Therefore, a total of 48 patients were included in this study, and their 28-day survival rate was 50%. Figure 1 shows the APACHE II score, SOFA score, fluid balance, use of aortic balloon pump or continuous arteriovenous hemofiltration, the number of patients receiving heart transplantation, and the number of patients who survived within 28 days and were discharged from the hospital. , ICU hospital stay and total hospital stay.
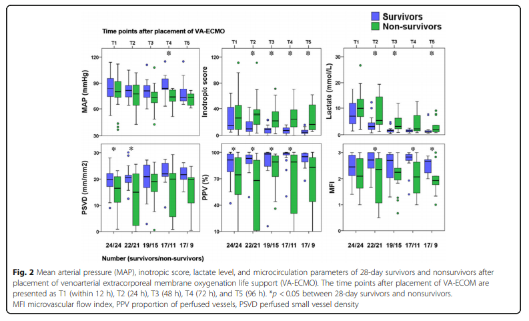
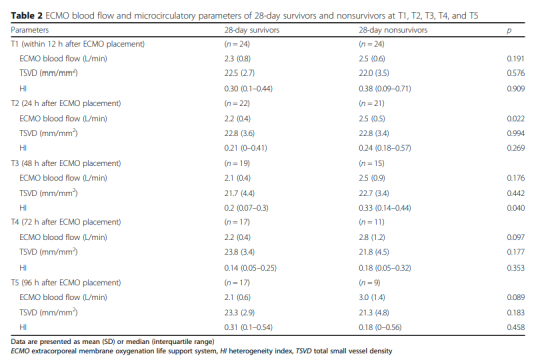
Hemodynamic parameters, vasoactive drug scores, lactate levels and microcirculation parameters at different time points
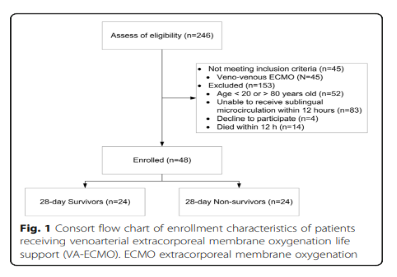
The hemodynamic parameters, vasoactive drug score, lactic acid level and microcirculation parameters at T2, T3, T4 and T5 are shown in Figure 2 and Table 2. At T1, there was no significant difference between MAP, vasoactive drug scores, and lactate levels in patients who died within 28 days and survivors, but PSVD and PPV in patients who died within 28 days were lower than those in survivors. The hemodynamic parameters of R0, R1, R2, R3 and R4, vasoactive drug score, lactic acid level and microcirculation parameters are shown in Figure 3. At R0, there was no difference in MFI scores between patients who died at 28 days and those who survived. At R1, the MFI scores of patients who died at 28 days were lower than those of survivors.
Prognostic test and subgroup survival analysis of 28-day mortality
Figure 4 shows the ROC curve used to analyze 28-day mortality. According to the ROC curve, the thresholds for APACHE II score, lactate level, PSVD and PPV are 22.5, 7.5 mmol/l, 16.2 mm/mm2 and 76.5%, respectively. Figure 5 shows the 28-day survival curve grouped by PSVD and PVD values at T1. The two subgroups with higher PSVD and PPV values had higher survival rates than the subgroup with the lowest PSVD and PPV values.
Discuss
This study shows that compared with those who survived within 28 days under the support of VA-ECMO, the microcirculation dysfunction of the dead patients is more severe. In addition, this study shows that PSVD and PPV at T1 can be used to predict the prognosis of such patients. In addition, patients were divided into three subgroups according to the 25th and 75th percentiles of PSVD and PPV at T1. The survival rate of the two subgroups with higher PSVD and PPV values was higher than that of PSVD and PPV. Subgroup.
This study found that the PSVD and PPV of patients who died at 28 days were lower than those of survivors at T1. However, MAP, vasoactive drug scores and lactate levels were not significantly different from those of surviving patients at T1. This is consistent with the view that microcirculation dysfunction may occur in normal circulation parameters. Therefore, we believe that MAP may not be the best or ultimate goal of resuscitation for patients with cardiogenic shock receiving VA-ECMO support.
However, monitoring microcirculation parameters may help predict its prognosis and provide information about whether tissue perfusion is sufficient. However, further studies are needed to prove the effect of improving microcirculation on the prognosis of patients. In addition, judging from the number of people, there are more deaths than surviving patients at 28 days. Fluid overload may cause and aggravate the degree of shock in patients who died at 28 days. Fluid overload may cause microcirculation exchange disorders, thereby reducing tissue oxygenation capacity.
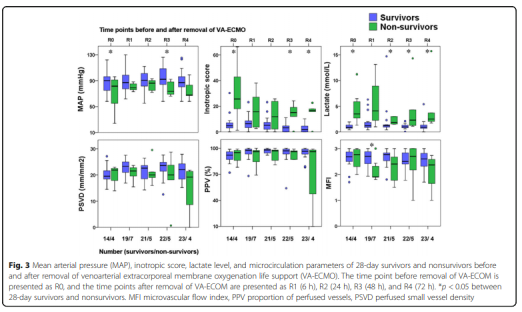
This study shows that compared with surviving patients, the microcirculation disorders in dead patients are more severe. This result is consistent with the findings of Kara et al. However, there are some differences between the findings of the two studies.
First, we found that patients who died at 28 days had higher APACHE II scores than those who survived. However, in the study of Kara et al., there was no significant difference between the APACHE II scores of dead and surviving patients.
Secondly, in this study, the PPV values of patients who died and survived at 28 days were lower than those reported by Kara et al.
Third, in this study, the ECMO auxiliary flow at T2 was higher in the dead patients than in the surviving patients. However, in the study of Kara et al., there was no significant difference in ECMO auxiliary flow levels between surviving patients and dead patients.
In addition, in the study of Karal et al., the auxiliary flow of VA-ECMO was higher than the auxiliary flow of VA-ECMO in this study. We believe that the reasons for the different conclusions of the two studies are as follows:
First, compared with Kara’s study, the number of patients included in this study is larger (48: 24).
Second, we measured the microcirculation parameters within 12 hours after VA-ECMO assistance, while in Kara’s study, we measured the above parameters within 24 hours after VA-ECMO assistance.
Third, the two studies have different definitions of the diameter of small blood vessels (<20μm vs <25μm). In addition, Kara et al. used the PSVD (<100μm) of all blood vessels to predict the survival rate based on the ROC curve.
Fourth, the two studies were targeted at different mortality rates (28-day mortality rate and ICU mortality rate).
In this study, the PSVD of R4 surviving patients was still lower than that of 70 healthy volunteers (21.8 (3.7) vs. 25.2 (2.3) mm/mm2, p <0.001). Persistent microcirculation disorders in patients receiving VA-ECMO assistance may be related to the primary disease, VA-ECMO-related inflammation, and hemolysis-related endothelial cell dysfunction. Persistent microcirculation disorders are associated with organ failure and death in patients with septic shock.
More research is still needed to explore the effect of persistent microcirculation disturbance on organ dysfunction in patients with VA-ECMO support. Before the VA-ECMO weaning, there was no significant difference in the MFI scores between the dead and surviving patients at 28 days. However, within 6 hours after VA-ECMO weaning, the MFI score of the dead patients was lower than that of the surviving patients. Therefore, reducing the auxiliary flow before VA-ECMO weaning needs further research on the change of MFI score.
This may help predict the microcirculation state after VA-ECMO is removed. Another related study compares the microcirculation parameters with the current expected parameters of VA-ECMO weaning. In addition, among the dead patients, the lactic acid levels at R0, R2, R3 and R4 were higher than those of the surviving patients. Therefore, lactic acid levels may provide more information before and after VA-ECMO weaning.
This study still has certain limitations. First, the mechanism of microcirculation disorder and its impact on mortality can vary from person to person due to the different primary diseases that cause cardiogenic shock, but the sample size of this study is too small to study such differences. Secondly, due to the death of some patients at T1, the number of patients who died at other time points decreased.
For the comparison of variables between dead and surviving patients at other time points, this study cannot detect the difference. In addition, this means that the test is not suitable for analysis of variance. These data provide preliminary information for the further study of microcirculation changes at other time points after VA-ECMO is started and weaned.
Third, the optimal cut-off point of microcirculation parameters may have been limited by the diameter of microvessels (<20μm or <25μm) or the observable microcirculation blood vessel range (only small blood vessels or total blood vessels). We suggest that cardiogenic shock caused by different etiologies may affect different types of microcirculation blood vessels. Other studies are needed to explore the optimal diameter of small blood vessels and the optimal range of visible microcirculatory vessels in different causes of cardiogenic shock.
Conclusion
This study shows that early microcirculation parameters can be used to predict the prognosis of patients with cardiogenic shock supported by VA-ECMO. At the same time, further research is needed to improve the survival rate of patients with cardiogenic shock supported by VA-ECMO by improving microcirculation.
Expert Comment:
It is well known that both sepsis and cardiogenic shock suffer from microcirculation dysfunction, and microcirculation dysfunction is closely related to the prognosis of the disease. Although ECMO support is provided, the key to successful treatment is whether ECMO support therapy can provide adequate oxygen supply for each organ.
This study used video microscope to observe the sublingual microcirculation within 12 hours after VA-ECMO assistance, and used PSVD, PPV, lactic acid level and other parameters to evaluate the 28-day mortality and survival rate of patients. Among the 48 patients included in this study, the PSVD and PPV of dead patients were lower than those of survivors at 12 hours after VA-ECMO assisted. PSVD and PPV were slightly better than lactate levels in predicting 28-day mortality; PSVD and PPV values The lowest subgroup has a lower survival rate.
This study shows that early microcirculation parameters can be used to predict the prognosis of patients with cardiogenic shock supported by VA-ECMO. Patients receiving VA-ECMO assistance have persistent microcirculation disorders that may be related to the primary disease and inflammation associated with VA-ECMO. Reaction and hemolysis-related endothelial cell dysfunction are related.
The study also found that MAP, vasoactive drug scores and lactate levels in patients who died at 28 days were not significantly different from those in surviving patients at T1. Therefore, we believe that MAP may not be suitable as the best or ultimate goal of resuscitation for patients with cardiogenic shock receiving VA-ECMO support. However, monitoring microcirculation parameters may help predict its prognosis and provide information about whether tissue perfusion is sufficient.
However, further studies are needed to prove the effect of improving microcirculation on the prognosis of patients. This study still has certain limitations. In the future, it is necessary to carry out more research to explore related mechanisms in depth and accumulate more evidence to guide clinical practice.
V-A ECMO support treatment on the microcirculation of patients
V-A ECMO support treatment on the microcirculation of patients
V-A ECMO support treatment on the microcirculation of patients
V-A ECMO support treatment on the microcirculation of patients
(source:internet, reference only)
Disclaimer of medicaltrend.org