How rabies virus spreads from periphery to the central nervous system?
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
How rabies virus spreads from the periphery to the central nervous system?
How rabies virus spreads from periphery to central nervous system? Controversy and latest developments. the official documents of the World Health Organization (WHO) also clearly stated that “rabies virus particles exclusively only travel along motor neuron axons (axons), and quickly reach the central nervous system by means of reverse transport, without being Ingested by sensory or sympathetic nerve endings.”
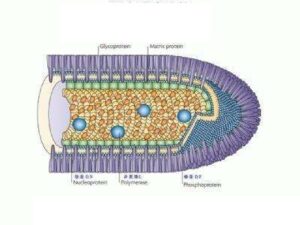
3 years ago, the official documents of the World Health Organization (WHO) also clearly stated that “rabies virus particles exclusively only travel along motor neuron axons (axons), and quickly reach the central nervous system by means of reverse transport, without being Ingested by sensory or sympathetic nerve endings.”
However, the latest edition (4th edition) of Rabies, the most authoritative and comprehensive large-scale academic monograph on rabies research in the world, published on May 5, 2020, raises doubts about the centrality of rabies virus to the CNS. Transmission occurs in the motor axons of the peripheral nerves, and may also occur in the sensory axons.
The World Health Organization (WHO) held another rabies expert consultation in Bangkok, Thailand in April 2017. The technical report of this consultation (“WHO Rabies Expert Consultation Third Report”, English version) was published in 2018. It was officially released on the WHO website on April 20.
Regarding the transmission of rabies virus (RABV) from the wound to the CNS after entering the human body, the report describes it as follows: RABV may replicate in muscles or other local tissues after exposure, and then pass through end plates and motor axons (Axons) reach the central nervous system. Virus particles use transport vesicles as carriers, exclusively only along motor neuron axons, and quickly reach the CNS in the reverse transport mode, without being taken up by sensory or sympathetic nerve endings.
On May 5, 2020, the latest edition (4th edition) of the large academic monograph “Rabies” was published. The book is hailed as the most authoritative and comprehensive encyclopedia on rabies research in the world. The book questioned the above issues and believed that the centripetal transmission of rabies virus to the CNS occurred in the motor axons of the peripheral nerves, and may also occur in the sensory axons.
Regarding how the rabies virus spreads from the periphery to the CNS, the book cites a large amount of experimental evidence to illustrate:
The centripetal transmission of rabies virus (RABV) to the CNS occurs in the motor axons of the peripheral nerves, and may also occur in the sensory axons (axons). Colchicine is a microtubule disrupting agent that has activity on the cytoskeleton structure containing microtubules, and is an effective inhibitor of rapid axon transport in the sciatic nerve of rats.
In the rat experiment, colchicine was applied locally to the sciatic nerve, and the fiber sponge was used to increase the concentration of the drug only locally, thereby avoiding systemic adverse reactions. It was found that the spread of RABV was prevented, which provides strong evidence for the spread of RABV from the peripheral inoculation site to the CNS through retrograde rapid axonal transport. The neurons of the human dorsal root ganglion were used in a separate cell culture system to prove that the retrograde transport of the virus occurred at a rate of 50 to 100 mm/day.
There is evidence that RABV phosphoprotein is a component of the ribonucleocapsid complex and can interact with dynein light chain 8 (LC8). Dynein LC8 is a component of cytoplasmic myosin V and dynein, which are involved in actin transport (important in the early stages of virus entry) and microtubule transport (rapid axon transport), respectively. It is speculated that the interaction between the phosphoprotein and dynein of RABV may play an important role in the axon transport of RABV.
However, studies conducted in young mice have shown that deleting the light chain region of dynein responsible for binding to the recombinant SAD-L16 virus (the recombinant virus contains the gene sequence of the SAD-B19 strain) leads to the emergence of mutant viruses, which proves that peripheral inoculation is effective The spread of viruses has only a small impact, and these viruses are still neuroinfectious and neurotoxic. Experiments have shown that RABV glycoprotein pseudotyped lentivirus (using equine infectious anemia virus as a carrier) enhances the transfer of viral genes to neurons by promoting retrograde axon transport. Therefore, RABV glycoprotein may play a more important role than phosphoprotein.
Other researchers used mouse and hamster models to prove that the motor neurons in the spinal cord and the primary sensory neurons in the dorsal root ganglia are involved in the spread of the virus at an early stage and at least almost simultaneously. After inoculation of the CVS strain in the masseter muscle, early infection can be observed in the trigeminal ganglion.
Studies using RT-PCR amplification indicate that trigeminal ganglion infection can be detected 18h after vaccination, which is earlier than the time detected in the brainstem (24h after vaccination). However, neither the simple cross-neuron tracing method using the CVS strain in rats nor the research in rhesus monkeys found early infection of primary sensory neurons.
Two days after the CVS strain was inoculated into the bulbospongiosus muscle of rats, the distribution of RABV antigen was limited to the motor neurons of the bulbospongiosus muscle on the ipsilateral spinal cord. One more day later (day 3 after vaccination), there is evidence that the antigen has transferred to the gray commissure of the back, the intermediate area and the interneurons of the sacral parasympathetic nucleus and the motor neurons of the external urethral sphincter ; At this time, no primary sensory neuron markers were found in the local dorsal root ganglia.
This study shows that in the spread of RABV to the central nervous system, motor pathways rather than sensory pathways are important. It is not clear whether the different results from earlier studies are due to different animal models, including the type of host and the route of vaccination.
The clinical manifestations of rabies obtained from dogs and bats are different. For example, the more common symptoms of rabies obtained from bats are tremor and myoclonus, while the more common symptoms of rabies obtained from dogs are fear of water and wind.
Although there are no experimental studies to assess the route of transmission, the spread of bat viruses is usually through very superficial damage (for example, penetration into the superficial skin). It is estimated that there will be significant differences between the host and the route of virus transmission.
Dog bites often involve deep skeletal muscle, which may explain, at least to some extent, why there are different clinical manifestations when infected with these RABV variants.
(source:internet, reference only)
Disclaimer of medicaltrend.org



