CAR-T is expected to cure Liver cancer Stomach cancer Pancreatic cancer
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
CAR-T is expected to cure Liver cancer Stomach cancer Pancreatic cancer
CAR-T is expected to cure Liver cancer Stomach cancer Pancreatic cancer. The three most difficult to treat cancers are no longer difficult! CAR-T Therapy goes all out, liver cancer, stomach cancer, and pancreatic cancer are saved!
Speaking of the three most difficult-to-treat cancers, the top three are undoubtedly pancreatic cancer, liver cancer, and gastric cancer.
Cancer can be said to be the biggest killer threatening human health at present! But even though medicine is already very advanced, but cancer has not been conquered! Of course, this does not mean that all cancers are very difficult to cure. As long as some thyroid cancer, breast cancer, bladder cancer, etc. are actively treated, the cure rate is almost all over 90%. There are also very difficult to cure cancers, almost all of them grow in people’s abdomen!
Speaking of the three most difficult-to-treat cancers, the top three are undoubtedly pancreatic cancer, liver cancer, and gastric cancer. These three types of refractory cancers are all digestive system tumors. For digestive system tumors, surgery is currently the most effective method, but once there is no indication for surgery, there is almost no particularly effective treatment. Therefore, save money to develop new treatment methods to solve this problem!
Chimeric antigen receptor modified T cells (CAR-T) are undoubtedly one of the most anticipated treatments. This technology has made great progress in the treatment of various malignant tumors in recent years, and has been successfully applied to the treatment of various hematological malignancies. In addition, it has also achieved certain curative effects in solid tumors such as liver cancer, gastric cancer, pancreatic cancer and other digestive system tumors. Therefore, CAR-T therapy is a hot research topic in recent years, and different targets for these cancers are being studied.
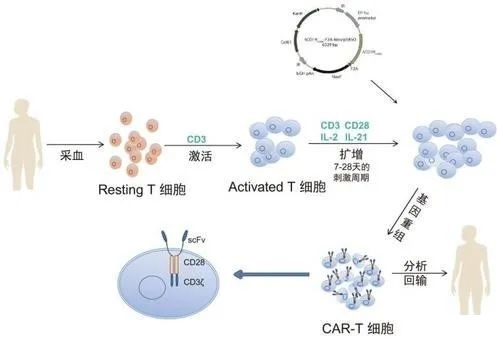
This article will summarize the clinical trial data of CAR-T cells in the treatment of liver cancer, gastric cancer, pancreatic cancer and other three refractory cancers for your reference.
Stomach cancer
China is a country with a high incidence of gastric cancer. According to the latest data from the China Cancer Registry, it ranks second in the incidence of malignant tumors in the same period. The overall prognosis of gastric cancer is poor. The 5-year survival rate is only 10%-30%. The 5-year survival rate of patients with advanced gastric cancer is less than 10%. The research results of first-line treatment of advanced gastric cancer show that the overall survival (OS) is continuously prolonged, but medium The survival period is still around 12 months.
The traditional treatment methods for advanced gastric cancer include surgery, radiotherapy and chemotherapy, but the cure rate for advanced gastric cancer surgery is low, and the curative effect of radiotherapy and chemotherapy is not good.
In one sentence, China’s gastric cancer treatment situation is grim!
China has developed the world’s first solid tumor CAR-T therapy targeting Claudin 18.2
According to reports, China’s CAR-T cell research and clinical treatment effects have caught up with American counterparts! In the field of solid tumors, China has achieved world-renowned achievements, and has developed the world’s first solid tumor CAR-T therapy targeting Claudin 18.2.
Claudin 18.2 (CLDN 18.2) is a gastric-specific membrane protein, which is considered a potential therapeutic target for gastric cancer and other cancer types. Based on this, Chinese researchers developed the first CAR-T cell targeting Claudin 18.2 in the world.
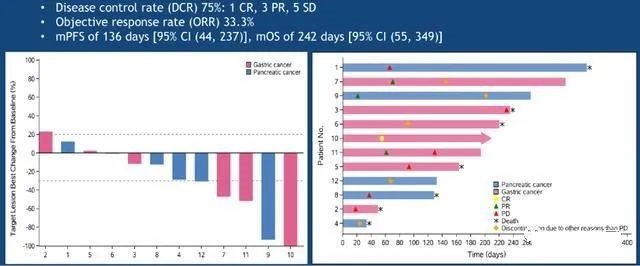
At the 2019 ASCO annual meeting, the clinical data update of CAR-Claudin 18.2 T cell treatment of gastric cancer/pancreatic cancer showed that 12 cases of metastatic adenocarcinoma (7 cases of gastric cancer and 5 cases of pancreatic cancer) were treated by targeting claudin 18.2 CAR T cells. Serious adverse events, treatment-related deaths, or severe neurotoxicity occurred. Among the 11 evaluated subjects: 1 case (gastric adenocarcinoma) had a complete remission; 3 cases (gastric adenocarcinoma, 2 cases of pancreatic adenocarcinoma, 1 case) had partial remission; 5 cases had stable disease; 2 cases had progressed; the overall objective remission rate was 33.3%.
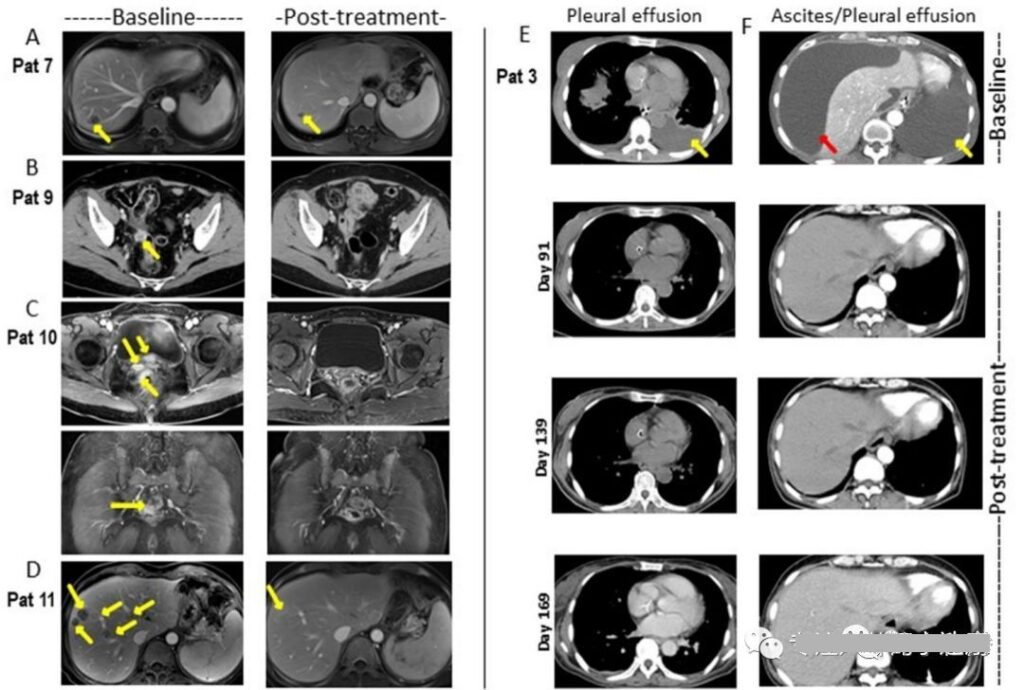
In addition, the preclinical research results of CAR-Claudin 18.2 T cells in the treatment of gastric cancer show that CAR-T cells targeting Claudin 18.2 can completely eliminate gastric tumors in a mouse model without off-target toxicity.
Liver cancer
The reason why liver cancer is difficult to treat is mainly due to its heterogeneity. Liver cancer is one of the most heterogeneous tumors. Clinically, patients with liver cancer who are in the same stage and have similar clinicopathological characteristics have significantly different recurrence and metastasis rates and survival rates after surgical resection, as well as their response to drug treatment.
Compared with the high mortality rate, the rate of early detection of liver cancer is very low. More than 90% of it is found to be in the advanced stage. At this time, the opportunity for surgical treatment has been lost, resulting in a 5-year survival rate of only 12%. The 5-year survival rate is as low as 3%.
Advanced liver cancer can also be saved! Chinese-only CAR-T clinical trials reappeared!
So today, the editor will introduce the world’s first exploratory CAR-T clinical trial for the treatment of advanced liver cancer with GPC3 targets completed by Shanghai researchers.
This breakthrough research result was published in the international authoritative journal “Clinical Cancer Research” on May 5. In two prospective phase I studies, adult patients with advanced GPC3 + HCC (Child -Pugh A) received autologous CAR-GPC3 T cell therapy after cyclophosphamide and fludarabine induced lymphatic clearance. The main purpose is to evaluate the safety of the treatment.
As of July 24, 2019, a total of 13 patients had received CAR-GPC3 T cells with a median value of 19.9×10^8. All patients were GPC3-positive, had received surgical treatment, local treatment or systemic treatment, and all carried hepatitis B virus (HBV).
Among them, 2 patients achieved partial remission (PR). The survival rates of all patients at 6 months, 1 year, and 3 years were 50.3%, 42.0%, and 10.5%, respectively. The median survival time (OS) was 278 days (39.7 weeks). ).
Typical cases
The survival times of these two patients were 20.5 months (Figure P13) and 44.2 months (Figure P3). One patient with stable disease has survived nearly 4 years so far. He had an inferior vena cava tumor thrombus and right at the time of enrollment. Atrial tumor thrombus and lymph node metastasis, after treatment, AFP (alpha-fetoprotein) maintains normal levels for a long time and survives.
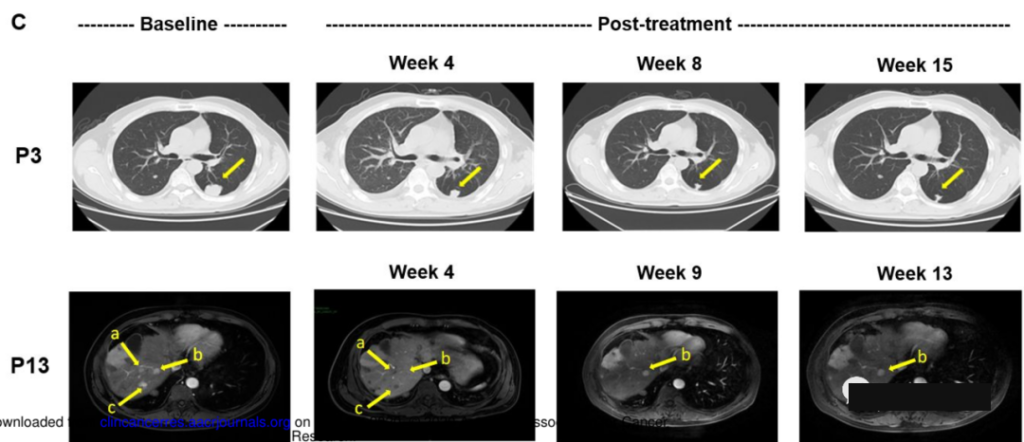
Experts believe that the results of the study show that GPC3 CAR-T has good safety and has shown preliminary effectiveness, and it is likely to become a new treatment for hepatocellular carcinoma.
Cancer-Pancreatic Cancer
Pancreatic cancer is known as the “king of cancer”, and its clinical features are cancers with a short course of disease, rapid disease progression, and rapid deterioration of the mortality rate. Because the pancreas is deep and concealed, it is difficult to find out even by ordinary B-ultrasound. Once the diagnosis is confirmed, it is in the late stage. The patients often show symptoms such as abdominal pain, nausea, weight loss, and ascites. They can only rely on chemotherapy and radiotherapy. Wait for surgery to relieve.
Improved GoCAR-T technical data is stunning
At the American Society of Clinical Oncology (ASCO) annual meeting held in June 2019, the data of a Phase 1/2 clinical trial for patients with metastatic pancreatic cancer expressing prostate stem cell antigen (PSCA) was announced. The company has developed a new and improved GoCAR-T technology-BPX-601, and its safety and activity data are quite positive. So far, BPX-601 has been well tolerated and no dose-limiting toxicity has been observed.
Most of the adverse events related to BPX-601 are mild to moderate and can be alleviated with or without supportive treatment.
Although the clinical trial design is relatively conservative, evidence of stimulating biological activity has been observed at the beginning of the study! Among the 13 patients with evaluable efficacy, 8 patients (62%) had stable disease, and 3 patients had tumors shrunk by 10% to 24%.
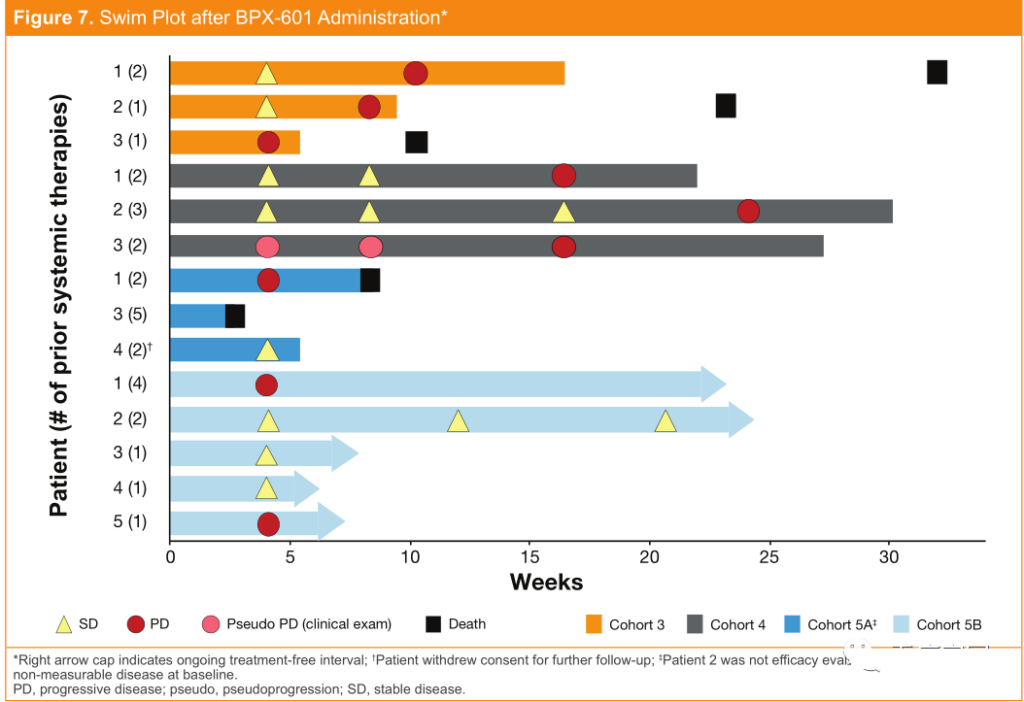
The efficacy of each patient after administration of BPX-601
Mr. Rick Fair, the chairman and executive officer of this company, stated that this therapy is more effective in design and is expected to break through the limitations of traditional CAR-T and better deal with solid tumors.
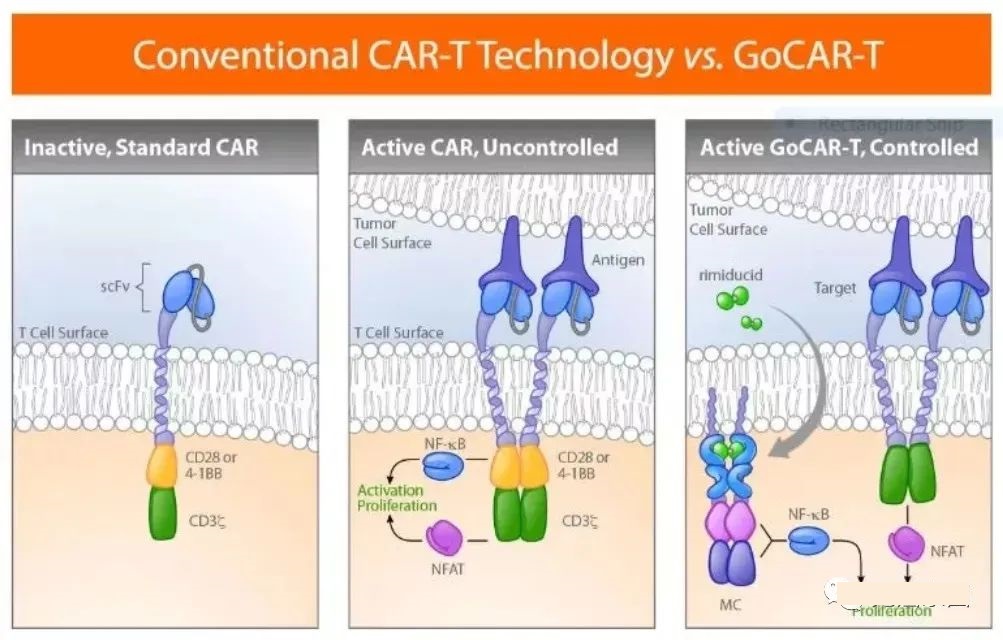
GoCAR-T technology allows CAR-T cells to be activated only in the presence of small molecule drugs
Although pancreatic cancer is a hard “hard bone”, researchers have expressed full confidence in it. When talking about future development plans, Mr. Rick Fair stated that he would continue to advance research on pancreatic cancer and plan to expand the research to include other PSCA-expressing tumors. Other drug candidates in the R&D pipeline include BPX-603 (a CAR-T cell product that combines an inducible MC activation switch and CaspaCIDe safety switch to target HER2), BPX-802 (dual switch GoCAR-T) and so on.
In addition to the international CAR-T breakthrough clinical studies reported above, the current clinical trial targets for the application of CAR-T in the treatment of pancreatic cancer include CD133, EGFR, EpCAM, MUC1, CEA, HER2, PSCA, and mesothelin.
Expert comments:
Digestive system tumors account for a large proportion of human tumors, especially in China, accounting for about half of all tumors. Therefore, it is urgent to find effective treatments for digestive system tumors. CAR-T cells targeting GPC3, CT041, CEA, CD133 and other targets provide potential treatments for liver cancer, gastric cancer, pancreatic cancer and other digestive system tumors.
In short, as CAR-T cells continue to evolve, safer and more efficient CAR-T cells will be successfully used to treat digestive system tumors in the near future, which will greatly extend the survival of patients!
CAR-T is expected to cure Liver cancer Stomach cancer Pancreatic cancer
(source:internet, reference only)
Disclaimer of medicaltrend.org



