Tisagenlecleucel to treat relapsed or refractory diffuse B-cell lymphoma
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
Tisagenlecleucel to treat adult relapsed or refractory diffuse large B-cell lymphoma
Tisagenlecleucel to treat relapsed or refractory diffuse B-cell lymphoma. Diffuse large B-cell lymphoma (DLB CL) is the most common non-Hodgkin lymphoma [1]. Although the condition of most patients responds well to the first-line immunochemotherapy combination containing rituximab, 10% to 15% of patients have primary refractory disease within 3 months after the start of treatment, and another 20% Recurrence to 35% [2].
Approximately 40% to 60% of patients with relapsed or refractory DLBCL respond to second-line chemotherapy; 50% of these patients continue to receive autologous hematopoietic stem cell transplantation, of which approximately 30% to 40% remain progression-free 3 years after transplantation [ 3-8]. For patients who cannot undergo high-level transplantation, dose chemotherapy and hematopoietic stem cell transplantation are used as second-line treatments, and the prognosis is poor. The median overall survival period is 4.4 months, and the 1-year and 2-year overall survival rates are 23% and 16%, respectively [8] . For the highly selected group of chemotherapy-sensitive patients who have relapsed after autologous transplantation, if the patient responds to chemotherapy and has a donor, allogeneic hematopoietic stem cell transplantation can be performed; however, this surgery has a high risk of treatment-related complications. The related mortality rate unrelated to disease recurrence is 23% at 1 year [9-13].
A recent retrospective study reviewed the results of 636 patients with primary refractory DLBCL or DLBCL recurrence within 12 months after autologous transplantation [5]. The response rate to next-line treatment was 26%, and the rate of complete response was 7%; the median overall survival was 6.2 months. These poor results have strengthened the need for new treatment options for patients with relapsed or refractory DLBCL.
The anti-CD19 chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel (formerly CTL019) has been shown to have a high level of curative effect and serious effects on children and young people with relapsed or refractory acute lymphoblastic leukemia. To a large extent reversible toxic effects [14,15]. After years of preclinical work and clinical development [14,15], tisagenlecleucel was approved by the Food and Drug Administration for use in such patients [16]. A high response rate was also observed in adult patients with stage IIa relapsed or refractory DLBCL. Single-center study: 3-month remission rate was 50%, 6-month complete remission rate was 43%; patients were followed up for a median of 28.6 months At 6 months, there was no recurrence in complete remission [17].
On the basis of these studies, a pivotal Phase 2 study was initiated to evaluate the safety and effectiveness of tisagenlecleucel in adult patients with relapsed or refractory DLBCL. JULIET is an international study conducted at 27 locations in 10 countries/regions in North America, Europe, Australia and Asia, involving cryopreserved leukocyte removal materials, centralized manufacturing, and global supply chains.
Test design
We conducted a single-group, open-label, multi-center, international phase 2 study of tisagenlecleucel for adult relapsed or refractory DLBCL. In order to be eligible for inclusion, patients must be 18 years of age or older and have received at least two previous treatments, including rituximab and an anthracycline. The patient either relapsed after autologous transplantation or is not suitable for autologous transplantation. We also included patients with DLBCL transformed from follicular lymphoma, and patients with high-grade B-cell lymphoma with MYC rearrangement plus BCL2, BCL6, or two gene rearrangements (ie double or triple-hit lymphoma) . Patients who have previously received CD19-directed therapy, have primary mediastinal DLBCL, have previously received allogeneic transplantation, or their DLBCL has active central nervous system involvement are excluded.
After providing written informed consent, all eligible patients underwent leukocyte removal; when the cryopreserved materials were transported to the manufacturing plant for manufacturing, the registration was completed. If necessary, bridging therapy is allowed. Before the infusion, the patient received a cycle of lymphocyte depletion chemotherapy (not required for patients with a white blood cell count ≤1000 cells/ml in the 1 week before the tisagenlecleucel infusion). For lymphatic depletion, patients can receive fludarabine (25 mg per square meter of body surface area) and cyclophosphamide (250 mg per square meter) for 3 days or bendamustine (90 mg per square meter) per day ) Treatment for 2 days.
Clinical endpoint
The primary endpoint is the best overall response rate (ie, the total percentage of patients with complete or partial response) determined by the independent review committee using the Lugano classification [18].
Secondary endpoints include the duration of response, overall survival, safety, and cytokinetic data for all patients receiving infusion; the evaluation of biomarkers is an exploratory analysis (see the methods section in the Supplementary Appendix, available in NEJM .org).
Statistical analysis is planned to conduct interim and main analysis for the top 50 and top 80 patients in the efficacy analysis set to ensure that 94% of the efficacy rejects the null hypothesis that the overall response rate is 20% or lower, and the basic response rate is 38% based on the assumption . It is planned to determine the P value in the interim analysis; only when the interim analysis does not show significance, the P value will be determined in the final analysis. This was accomplished by using a two-view Lan-DeMets group sequence design with O’Brien-Fleming type boundaries and precise confidence intervals, with a one-sided cumulative significance level of 0.025. The Kaplan-Meier curve is used to check the survival distribution.
Results
Patients
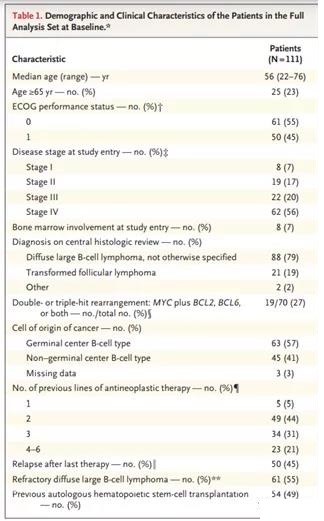
From July 2015 to the data deadline of December 8, 2017, a total of 238 patients were screened and 165 were included (Figure 1). Of the included patients, 111 (67%) received the infusion: 95 in the main cohort, 16 in cohort A (Figure 1 and the methods section in the Supplementary Appendix); 4 patients (2%) were in Waiting for infusion at the time of analysis. Patients receive infusions in an inpatient or outpatient setting. The median time from enrollment to infusion was 54 days (90% of patients received an infusion between 30 and 92 days after enrollment). The median time from infusion to data cutoff was 14 months (range, 0.1 to 26 months). The baseline characteristics of the included patients and those who received the infusion were similar (Table 1 and Table S3 in the Supplementary Appendix); however, the physical status of patients who did not receive the infusion was often lower than that of the patients who received the infusion, and among those who did not receive the infusion , A greater proportion of DLBCL was not effective for the last treatment they received before enrolling in the group.
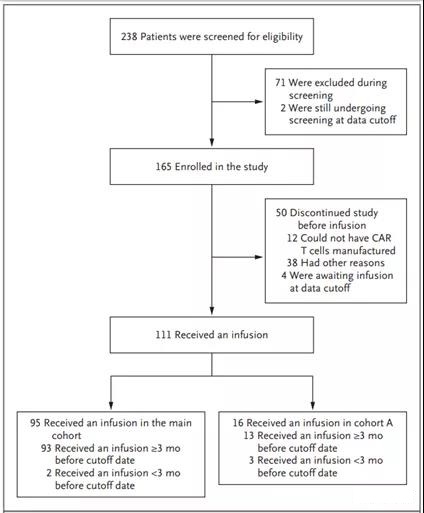

Before reinfusion, 92% of patients received bridging therapy, including rituximab (54%), gemcitabine (40%), etoposide (26%), dexamethasone (25%), and cisplatin (19 %) and cytarabine (19%)), as well as newer drugs such as ibrutinib (9%) and lenalidomide (7%). A total of 103 patients (93%) received lymphocytic depletion chemotherapy (73% received fludaabine-cyclophosphamide combination therapy, 20% received bendamustine). All 111 patients received a single infusion of tisagenlecleucel (median dose, 3.0×108 CAR-positive live T cells; range 0.1×108 to 6.0×108) (Table S4 in the Supplementary Appendix).
Curative effect
In the interim analysis, the null hypothesis (P<0.001) regarding the primary endpoint (ie, the best overall response rate ≤20%) was rejected [22,23]. Among the 93 patients in the efficacy analysis group, who had 3 months or longer follow-up or stopped participating in the study 3 months ago, the best overall response rate was 52% (95% confidence interval [CI], 41 to 62 ): 40% of patients had complete remission and 12% of patients had partial remission (Table S5 in the Supplementary Appendix). The overall and complete remission rates were 38% and 32% in the 3rd month, and 33% and 29% in the 6th month. A high degree of agreement (85%) between local and central assessments was found.
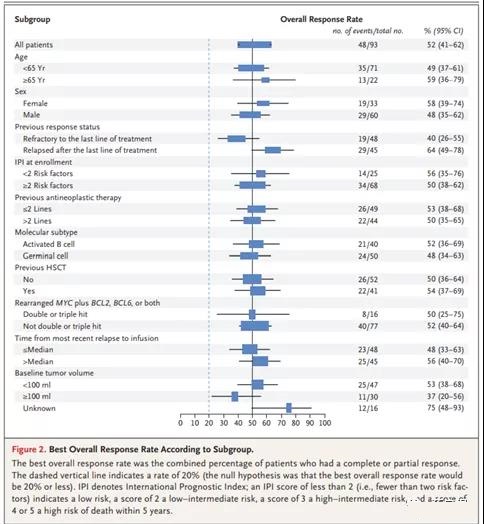
According to the type of lymphocyte clearance therapy received, there was no significant difference in remission rate (Table S6 in the Supplementary Appendix). Univariate analysis showed that the main demographic and prognostic subgroups (including subgroups based on disease response) were homogeneous and consistent treatment effect. Previous treatment (Figure 2 and Supplementary Appendix in Figure S1).
Of the 37 patients in complete remission, 16 patients had stable disease (4 patients) or partial remission (12 patients) 1 month after the infusion, with a median time of 2 months (range 1 to 17). 54% of patients (13 of 24) changed from partial remission to complete remission, including 2 patients whose initial response was confirmed by positron emission tomography 15 to 17 months after treatment. Among the 35 patients in remission at month 3, the estimated probability of maintaining remission at month 12 is 81% (95% CI, 63 to 91). In the intention-to-treat analysis that included all 165 enrolled patients, including patients who stopped participating before the infusion of tisagenlecleucel (mainly due to disease progression and death), the overall response rate was 34% (95% CI, 27 to 42).
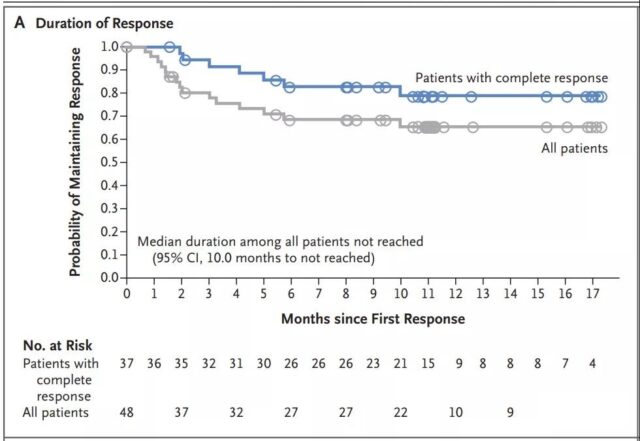
The median duration of response has not been reached (95% CI, less than 10 months); however, 79% (95% CI, 60 to 89) of patients in complete remission and 65% (95% CI, 49 to 78) in remission The patient is expected to be relapse-free for several months at 12 months before remission (Figure 3A and Figure S1A in the Supplementary Appendix). A long-lasting response of up to 18.4 months was observed after the infusion. No patients were transplanted when they responded. Six unresponsive patients underwent hematopoietic stem cell transplantation (5 underwent allogeneic transplantation and 1 underwent autologous transplantation followed by allogeneic transplantation).
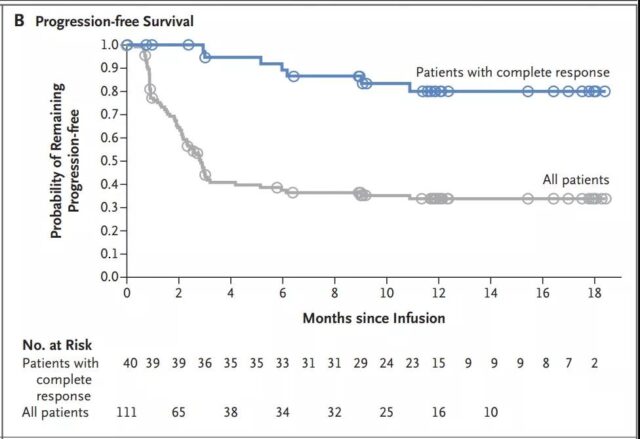
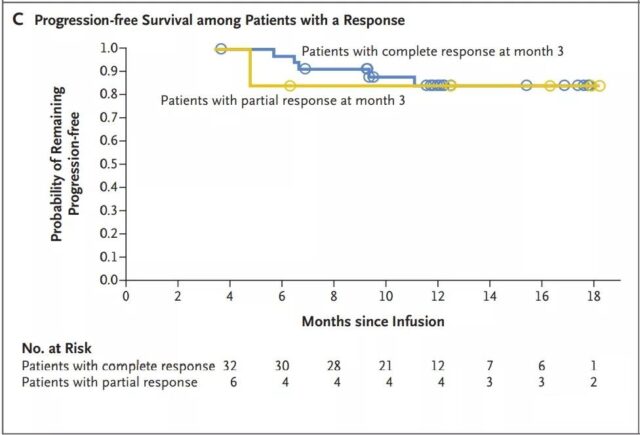
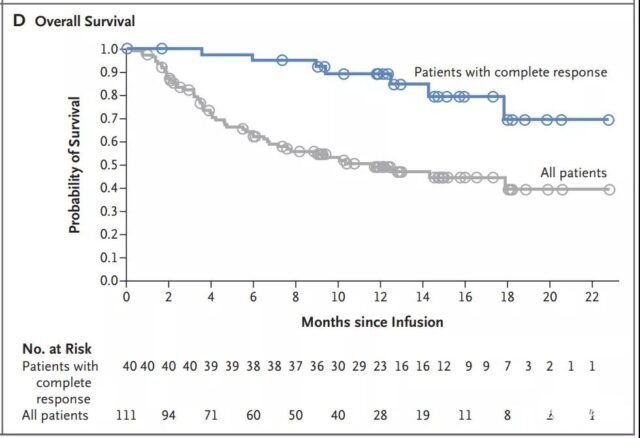
The median progression-free survival of patients with complete remission has not yet been reached (Figure 3B); among patients with complete or partial remission at 3 months, the progression-free survival rate at 12 months is estimated to be 83% (Figure 3C). The median overall survival of patients receiving the infusion was 12 months (95% CI, 7 months to not reached) (Figure 3D and Figure S1B in the Supplementary Appendix). In all patients, the estimated survival probability at 12 months was 49% (95% CI, 39 to 59), and 90% (95% CI, 74 to 96) for patients in complete remission. In an intention-to-treat analysis that included all 165 registered patients, the median overall survival from registration was 8.3 months (95% CI, 5.8 to 11.7), and the estimated survival probability at 12 months was 40% (95% CI, 32 to 49) (Figure S2 in the Supplementary Appendix).
Tisagenlecleucel expansion and durability
Tisagenlecleucel’s similar average in vivo amplification and concentration-time curve, measured by the transgene level, the median time to reach the maximum transgene level, and the average area under the concentration-time curve from day 0 to day 28 (AUC0-28d) In the patients who responded and those who did not respond, no significant effect of exposure on clinical outcomes was observed (Table S7 in the Supplementary Appendix).
In patients with a durable response, sustained CAR transgenic levels were observed for up to 2 years after infusion (Figure S3 in the Supplementary Appendix). There is no obvious relationship between dose and maximum in vivo expansion, and clinical responses have been observed in a wide range of doses [24].
Safety
The most common adverse events of any grade were cytokine release syndrome (58%), anemia (48%), fever (35%), decreased neutrophil count (34%), decreased platelet count (33%), white blood cells Count reduction (33%) and diarrhea (32%) (Table S8 in the Supplementary Appendix). Grade 3 or 4 adverse events of special concern in the first 8 weeks after infusion (Table 2) include cytokine release syndrome (22% of patients, according to the University of Pennsylvania Grading Scale 19), and unresolved cytopenias on day 28 (32%) (for information on different types of long-term cytopenias, see Table S9 in the Supplementary Appendix), infections (20%), neurological events (12%), and febrile neutropenia ( 15%).
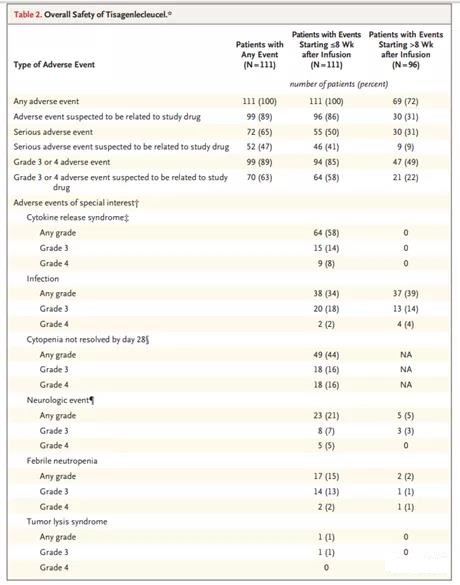
The median time from infusion to the onset of cytokine release syndrome symptoms was 3 days (except for one patient, all patients had onset within 9 days), and the median duration was 7 days (range, 2 to 30). The median time to grade 3 or 4 cytokine release syndrome is 4 days (range, 2 to 8 days); 97% of cases have been resolved through data cut-off. Overall, 14% of patients received tocilizumab, and 10% of patients received both tocilizumab and glucocorticoids. No patient received more than two doses of tocilizumab (5% received one dose and 9% received two doses). Patients with cytokine release syndrome received supportive treatment, including oxygen inhalation (24%), endotracheal intubation (7%), high-dose vasopressor 19 (6%) and dialysis (5%); 24% were admitted to severe illness ICU. 6% of patients also developed cytokine release syndrome infection.
21% of patients had neurological events of any grade within 8 weeks after the infusion; the median onset time was 6 days (range, 1 to 17 days), and the median duration was 14 days. Eight weeks or less after the infusion, 20% of patients experienced headache (not classified as a neurological disorder). A total of 13 patients (12%) had grade 3 or 4 events, most of which had been resolved by supportive treatment in accordance with local guidelines (such as glucocorticoids) at the time of data cut-off. Nine patients with grade 3 or 4 neurological events also had cytokine release syndrome. No fatal brain edema was observed.
Before tisagenlecleucel infusion, only one patient had a normal peripheral blood CD19+ B cell count (normal range, 80 to 616 per cubic millimeter); most of the CD19+ B cell count was below the lower limit of quantification (0.2/mm) (Table in Supplementary Appendix) S10). After the infusion, the CD19+ B cell counts of 6 patients who sustained complete remission returned to the normal range (5 patients >6 months after the infusion, and one patient at the 3rd month). Intravenous immunoglobulin is at the discretion of local researchers; 30% of patients receiving tisagenlecleucel infusion receive intravenous immunoglobulin therapy after the infusion.
Three patients died within 30 days after the infusion, all of them died of lymphoma progression. The researchers blamed tisagenlecleucel for death after the infusion.
Biomarkers
Quantitative immunofluorescence analysis was performed on tumor tissues before infusion to determine the expression of CD19; CD3, PD-1, and PD-L1; and CD3, TIM3, and LAG3. Samples were collected 1 month to 1 year (60 of 82 patients) or more than 1 year (22 of 82 patients) before the infusion of tisagenlecleucel (Table S11 in the Supplementary Appendix). Pairs were observed in tumor samples with clear CD19 expression (best overall response rate, 49%; 95% CI, 34 to 64) and low or negative CD19 expression (best overall response rate, 50%; 95% CI) tisagenlecleucel’s response, 29 to 71) (Figure S4A and Table S12 in the Supplementary Appendix).
We found that there was no significant difference in the median or average PD-1–PD-L1 interaction score between the groups with the best overall response (see the Supplementary Appendix to the Methods section) or the percentage of cells expressing immune checkpoint-related proteins (PD-L1 The percentage of total cells that are positive for PD-1, LAG3, or TIM3 and the percentage of total CD3 T cells expressing PD-1, LAG3 or TIM3) are at baseline (Figure S4B to Figure S4E in the Supplementary Appendix, data not shown).
However, the 5 patients with the highest PD-1-PD-L1 interaction score either did not respond to tisagenlecleucel (4 patients) or relapsed by month 3 (1 patient). Similarly, 11 patients had the highest percentage of LAG3+ T cells (in total T cells) that did not respond to tisagenlecleucel (7 patients) or relapsed within 3 to 6 months (4 patients, 2 after a complete response, 2 After partial reaction) (see Methods section and Table S13 in the Supplementary Appendix). In patients with the highest percentage of PD-1+ T cells or TIM3+ T cells (in total T cells), no significant non-response or early recurrence was observed. .
The study showed that in adult patients with relapsed or refractory DLBCL who had undergone extensive pretreatment, the response rate and duration of tisagenlecleucel treatment were high. In terms of the primary endpoint, the results were significant, with the best overall response rate being 52% [23]. 4 patients with stable disease and 12 patients with partial remission improved to complete remission within 1 month, with a median of 2 months. At 3 months, the complete remission rate and partial remission rate were 32% and 5%, respectively, and lasted for 6 months, which indicates that the remission at 3 months is usually long-lasting. For patients with double-hit lymphoma, the remission rate is 50%, and the complete remission rate is 25%. The long-term persistence of tisagenlecleucel is up to 2 years.
Responsive patients have a longer duration of CAR transgene levels than non-responding patients; however, there is no evidence of a dose response or exposure-response (exposure measured as AUC0-28d or peak cell expansion) relationship. A retrospective SCHOLAR-1 study showed that the comprehensive complete remission rate with standard care therapy was 7%, and the median overall survival was 6.2 months [5]. Therefore, our findings indicate that tisagenlecleucel has the potential to improve the prognosis of patients with relapsed or refractory DLBCL.
Although 58% of patients developed cytokine release syndrome, a grade 3 or 4 event (based on the University of Pennsylvania Grading Scale to manage cytokine release symptoms or hemodynamic complications [or both] Interventions to define) occur in 22% of patients and respond to tocilizumab in most cases. By monitoring the patient for fever, which is the first symptom of cytokine release syndrome, and ensuring that the syndrome is managed by appropriately trained field personnel using protocol-specific algorithms [20], this severe toxic effect is achieved Control, no fatal incidents. No deaths were attributed to tisagenlecleucel, cytokine release syndrome, or cerebral edema.
Before the infusion, most patients were depleted of B cells due to previous treatment with rituximab. Most patients with measurable pre-infusion levels of rituximab have B cell counts below the limit of quantification (0.2/mm) (Table S10 in the Supplementary Appendix). In 83% of lymphoma patients, rituximab causes circulating and tissue-based B cell depletion for up to 9 months. 25 Monitor immunoglobulin levels throughout the course of treatment. Conduct the study at the time points specified in the protocol. Most patients (96%) had a history of rituximab-based treatment before entering the study. As expected, 74%, 49%, and 63% of patients had decreased IgG, IgA, and IgM levels, especially Before infusion. No analysis was performed to correlate infection with immunoglobulin levels. Although it has been reported that treatment with tisagenlecleucel can cause continuous depletion of B cells in pediatric patients with acute lymphoblastic leukemia [17,26,27], in patients with complete remission after tisa genlecleucel, B cells continue to reappear and over time Immunity levels have improved over time. According to reports, adult patients with lymphoma have longer treatment times and longer follow-up times [17]. Further follow-up is needed to evaluate the recovery of B cells and immunoglobulins in adult patients in this study.
Our research design reflects the real world scenarios of CAR T cell therapy candidates. The use of leukocyte removal with cryopreservation and bridging therapy allows flexibility in scheduling and maintaining disease control, and the use of centralized manufacturing and a global supply chain allows the international distribution of tisagenlecleucel to patients.
A total of 30% of the selected patients discontinued their participation in the study without receiving an infusion, mainly due to disease progression and death; due to manufacturing failure (mainly due to slow cell growth), 7% of the selected patients did not receive an infusion. The discontinuation before infusion was mainly due to the limited production capacity at the beginning of this study, which is a reversible logical factor. KTE-C19 is an anti-CD19 CAR T-cell drug with a CD28 costimulatory domain. It is evaluated as a hospitalized treatment for patients with relapsed or refractory DLBCL in ZUMA-1, a key single-group phase 2 study. 28 In an interim analysis, the study involved 51 DLBCL patients who were followed up for at least 3 months.
The response rate was 76%, 92% of the responses occurred within the first month, and the complete response rate was 33% at the 3.29 month. In the main analysis (involving 77 patients), the complete remission rate at the 6th month was 31%, and the partial remission rate was 5% [30]. Cytokine release syndrome occurred in 93% of patients (13% of patients ≥ grade 3, according to the Lee Grading Scale) [28,31]. Four patients had sustained cytokine release syndrome events at the time of death [28]. 64% of patients (28% ≥ grade 3) had neurological toxicity. CAR T cell expansion is significantly correlated with response [28]. Although there are differences in the patient population, the study design and CAR structure precludes direct comparisons between studies. The results of this study combined with our findings indicate that CD19-directed CAR T-cell therapy provides a high rate of long-lasting response, although Seriously, the security profile is a bit different.
Theoretically, the low or negative pre-infusion expression of CD19 may be the reason for the failure of tisagenlecleucel in some unresponsive patients. In an exploratory analysis to assess relative CD19 expression in pre-infusion biopsy, it was found that the response rate between the CD19 expression subgroup and the CD19 expression low or negative subgroup was similar, and responses were observed at all CD19 expression levels; these findings indicate At the immunohistochemical level, low or undetectable CD19 expression may be sufficient to make tisagenlecleucel treatment effective (Figure S4A in the Supplementary Appendix).
Exploratory biomarker analysis showed that in the best overall response group or patients with improved response over time, the pre-infusion expression of inhibitory immune checkpoint protein in tumor cells or tumor microenvironment was associated with the eventual lymphoma progression There is no difference between patients. However, the few patients with the highest PD-1–PD-L1 interaction scores and the subgroup with a high proportion of LAG3+ T cells (among the total T cells present) either did not respond to tisagenlecleucel or responded quickly 3 to 3 Disease progression within 6 months. It needs to be emphasized that these are preliminary observations and await further investigation.
The high and long-lasting response rate observed with tisagenlecleucel treatment is promising. However, it should be noted that the follow-up time is short, and the possibility of long-term toxic effects needs further analysis. Side effects such as cytokine release syndrome can be serious and even life-threatening; however, most patients are managed through supportive measures and cytokine blockade. There are few treatment options for patients with relapsed or refractory DLBCL who are not suitable for high-dose therapy and hematopoietic cell transplantation or for such unsuccessful treatments. For these patients, tisagenlecleucel shows promise, which needs to be confirmed by longer follow-up and larger studies.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



