Trial results of the safest drug for the treatment of COVID-19 infections
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
Trial results of the safest drug for the treatment of COVID-19 infections
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- The viral load of Delta variant is 1260 times the original COVID-19 strain
Trial results of the safest drug for the treatment of COVID-19 infections. “So far the safest drug for the treatment of COVID-19 infections!” The clinical trial results came out!
Introduction: The content of the article issued on August 8 introduced the efficacy test of fenofibrate against the new coronavirus in vitro, and it was found that the effect was as high as 70%. Recently, the results of clinical trials of the drug in Israel were also released, and the results were cheering, and the research team believes that the drug is currently the safest drug that can be used to treat COVID-19 infections! At the same time, more pharmacological effects of the drug against the new coronavirus have been discovered, which will also promote research on fighting against virus variants.
The new coronavirus (SARS-CoV-2) has infected more than 165 million people worldwide and caused nearly 3.5 million deaths. Recent vaccination efforts have also been hindered by the emergence and resistance of multiple coronavirus variants. Although the infection usually produces mild symptoms, in some patients, it may develop into a severe inflammatory COVID-19disease that requires medical intervention.
Recently, the team of Professor Yaakov Nahmias of the Hebrew University of Jerusalem, Israel discovered that the new coronavirus causes abnormal accumulation of lipids, which can cause severe inflammation in the process of lipotoxicity. .
The team identified the lipid-lowering drug TriCor (Tricor) fenofibrate as an effective antiviral drug last year, which showed significantly reduced lung cell damage in the laboratory and prevented virus replication. Later, the results were confirmed by multiple international research teams.
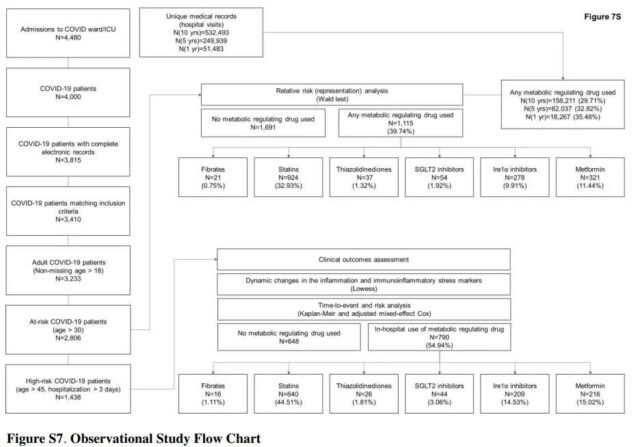
Observation Research Flow Chart Source: 10.21203/rs.3.rs-770724/v1
To support the initial in vitro experimental findings, an observational study was first conducted in multiple clinical centers in Israel in October last year. The study analyzed 3,233 Israeli patients who were hospitalized due to the COVID-19 infection and found that the effect of the drug supports the results of the in vitro study. Patients taking fibrates have significantly lower immune inflammatory markers and recover faster. The comparative epidemiological analysis from the European and American cohorts also confirmed this.
The team subsequently initiated an interventional clinical study at the Barzilai Medical Center in Israel with the support of Abbott Laboratories to treat patients with severe COVID-19 (COVID-19).
Today, the Hebrew University of Jerusalem team is reporting the results of an interventional open-label clinical study led by Nahmias and coordinated by Professor Shlomo Maayan, the head of the Barzilai Infectious Diseases Department. The results are promising.
The research team has recently published the research results in the journal “Research Square Biological Sciences” and is currently undergoing peer review. The research paper is titled “Metabolic Regulation of SARS-CoV-2 Infection”.

Screenshot of official website paper
In this single-arm (that is, there is no special control group), open label (that is, no blinding, and both parties are aware of the actual administration situation) study, 15 severely hospitalized patients with COVID-19 pneumonia received oxygen support. In addition to standard care, the patient also received a 10-day 145 mg/day TriCor (fenofibrate) treatment, and continued monitoring of disease progression and outcome.
Exciting results
Compared with control patients who were admitted to the hospital during the same period and received standard care treatment, patients who received fenofibrate treatment had a rapid reduction in inflammation and a significantly faster recovery.
“The results are shocking,” Nahmias shared. “Progressive inflammation markers are a sign of the deterioration of COVID-19, but they declined within 48 hours after treatment. In addition, 14 of 15 critically ill patients no longer need oxygen support within one week of treatment, and historical records show that it is extremely large. Most critically ill patients receiving standard care treatment require long-term respiratory support,” he added. These results bring hope because the FDA approved TriCor (fenofibrate) for long-term use in 1975, and it has a strong record of safe use. “There is no panacea,” Nahmias emphasized, “but fenofibrate is a drug that is far safer than other drugs proposed for the treatment of COVID-19, and its mechanism of action is unlikely to be mutation-specific (that is, it will not It is invalid for virus variants).”
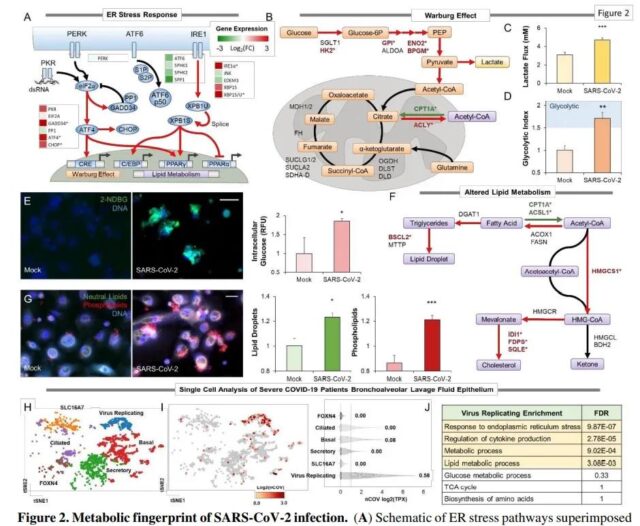
New Coronavirus Infection Metabolism Fingerprint Source: 10.21203/rs.3.rs-770724/v1
“All patients were discharged less than a week after the start of treatment and completed 10 days of treatment at home, and there were no reports of drug-related adverse events,” Maayan pointed out. “In addition, during the 4-week follow-up period, fewer patients reported side effects of COVID-19,” he added. These preliminary findings are expected to alleviate the huge health burden borne by patients who survive the acute phase of COVID-19 pneumonia.
Mechanism effect
Viruses are highly efficient metabolic engineers. They actively reconnect with host metabolic pathways to support their life cycle and are powerful metabolic targets for intervention. The study mapped the metabolic response of lung epithelial cells in primary cultures and samples from patients with COVID-19 to COVID-19 infection. Batch and single cell analysis showed that virus replication induces endoplasmic stress and lipid accumulation. Protein expression screening also showed that viral proteins, even in the absence of replication, are involved in mediating this metabolic reaction.
Drug screening based on metabolism showed that fenofibrate reversed lipid accumulation and prevented the replication of the new coronavirus. The research data shows that elevated lipid metabolism is the basis of key aspects of the pathogenesis of COVID-19, which indicates that pharmacological lipid metabolism regulation should be strongly considered for the treatment of coronavirus infections.
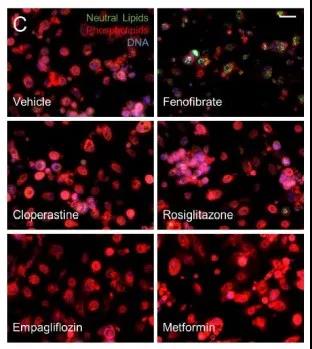
Therapeutic targets of new coronavirus-related host metabolic pathways
Source: 10.21203/rs.3.rs-770724/v1
Trials and recruitment are ongoing
The researchers emphasize that although the results are very promising, only randomized placebo-controlled studies can be used as the basis for clinical decision-making. “We have entered the second phase of the study and are actively recruiting patients,” Nahmias explained, noting that two Phase III studies are being conducted in South America, the United States (NCT04517396) and Israel (NCT04661930).
Related articles
In the frontiers in Pharmacology journal on August 6, 2021, a research paper titled “The Hyperlipidaemic Drug Fenofibrate Significantly Reduces Infection by SARS-CoV-2 in Cell Culture Models” was published, describing the drug fenofibrate (Fenofibrate) can reduce 70% of new coronavirus infections in cell culture dishes.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



