The review of oncolytic adenovirus therapeutic vectors
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
The review of oncolytic adenovirus therapeutic vectors
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The review of oncolytic adenovirus therapeutic vectors.
Adenoviruses have high genetic stability and low pathogenicity. They are relatively easy to produce with high titer and purity, which makes it widely used in various fields from gene therapy, tumor therapy to vaccine development.
Oncolytic adenovirus is a type of conditionally replicating adenovirus. It can replicate and lyse tumor cells relatively specifically in tumor cells. After releasing the progeny virus, it infects surrounding tumor cells and destroys the tumor through the cascade amplification effect, thereby obtaining better results. Good effect.
In recent years, oncolytic adenovirus therapy has become more and more important as a new type of treatment for various cancers.
Recently, Klaus Mantwill et al. published a review in the International Journal Of Molecular Sciences , expounding in detail from the convenience of genetic engineering strategies, foreign gene expression and immune system stimulation.

Four replication conditions in clinical trials
The adenovirus genome contains the early-expressed E1~E4 genes related to adenovirus replication and the late-expressed L1~L5 genes related to the assembly of adenovirus particles .
E1 deleted adenoviruses are considered to be replication-defective and are used as shuttle vectors in gene therapy or vaccination for gene therapy and vaccine immunization.
May be conditional promoter E1 region genes in tumor cells for adenovirus replication is called conditionally replicating adenovirus ( C RAd ), and also referred to in clinical oncolytic adenovirus (oncolytic Adenovirus)
Adenovirus vectors generally have four strategies to achieve conditional replication: E1A specific promoter regulation, E1A CR2 deletion, E1A 13S CR3 deletion and E1B-55K deletion.
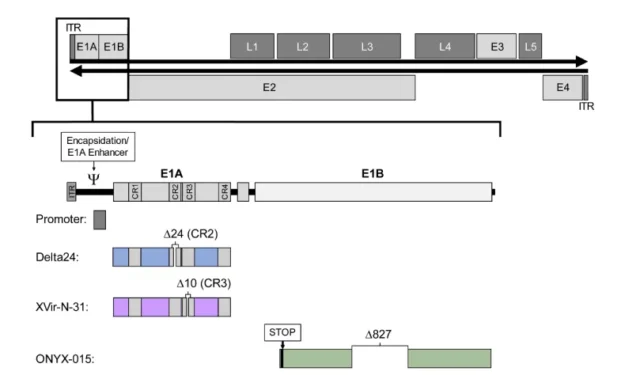
The picture shows the structure of the wild-type adenovirus serotype 5 genome
01. Specific promoters control E1 region to regulate virus replication
Tumor-specific promoters can cause high expression of specific genes.
Tumor or tissue-specific promoters can control E1A-mediated virus replication so that it can only be expressed in tumor cells, but is low or not expressed in normal cells.
Most of the foreign promoters are inserted into the E1A regulatory region, without any deletion or a small amount of deletion , such as CRAd-Survivin-pk7 and CG0070 .
02. E1A delete 24bp
In non-replicating cells, retinocytoma protein (pRB) can bind to gene regulatory protein E2F, thereby inhibiting cell proliferation.
The CR2 region of the adenovirus E1A protein also interacts with pRB, E2F is released and the virus replicates.
The deletion of adenovirus E1A CR2 prevents the combination of E1A and pRB, and the virus cannot release E2F in normal cells, nor can it replicate. In tumor cells with mutations or disorders of pRB, E2F is no longer negatively regulated by pRB and can activate viral gene transcription to replicate.
03. E1A delete 13s
Adenovirus E2 promoter can be divided into early and late promoters, used to regulate the E2 transcription unit, the late E2 promoter region contains Y-box, the binding site of transcription factor YB-1 (Y-box binding protein-1).
In normal adult cells, YB-1 is either not expressed or only located in the cytoplasm. Since E1A 13S is essential for the transport of YB-1 from the cytoplasm to the nucleus, adenoviruses lacking E1A13S expression have replication defects in normal cells. Such as: Dl520, Ad-Delo3-RGD (XVir-N-31)
04. Delete E1B55KD in E1 area
The replication of adenovirus in cells activates the TP53-mediated apoptosis pathway.
The E1B region of the adenovirus genome encodes a 55kD protein (E1B55K), which binds and inactivates TP53. Adenoviruses lacking E1B-55K will not be able to replicate effectively in cells with normal TP53 function, while they can replicate and lyse killer cells in large numbers in cells with TP53 gene mutation, deletion or inactivation.
Such as Dl1520 (ONYX-015), Oncorine (H101)
Oncolytic adenovirus and immune system
In normal non-cancer cells, the oncolytic adenovirus cannot replicate during infection, leaving the cell unaffected. In tumor cells, the virus can successfully replicate after infection, resulting in the production of more virus progeny, and ultimately induce immunogenic cell death (ICD).
Cell lysis results in the release of viral progeny, pathogen-associated molecular patterns (PAMP), damage-associated molecular patterns (DAMP), and tumor-associated antigens (TAA) into the tumor microenvironment (TME). The released offspring of the virus further infect uninfected tumor cells and continue to spread the virus.
The immunostimulatory molecules released into the TME attract immune cells to the tumor and lead to the activation of the tumor’s innate immunity and adaptive immunity.
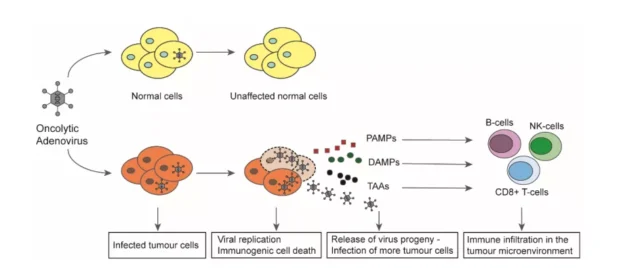
The picture shows the mechanism of action of oncolytic adenovirus in cancer cells.
Modified adenovirus vector to improve treatment effect
In addition to tissue-specific replication, a vector system that can improve the oncolytic and cytotoxic properties of adenovirus has also been developed.
The function of the product encoded by E1B-19K inhibits the death of infected cells and allows the virus to replicate.
The deletion of 19K can induce tumor cell apoptosis through multiple channels and enhance the virus transmission ability.
01. Insert foreign gene into adenovirus vector
In addition to ensuring cancer-specific replication, genes that promote oncolysis or stimulate the immune system have also been introduced.
The expression cassette is usually designed with an exogenous promoter, and the ribosome entry site, splice acceptor site or self-splicing polypeptide 2A can be used to regulate the expression of exogenous genes.
Another method is to regulate the inserted transgene through the adenovirus E3 promoter and E3 polyadenylation site.
In the E3 region, the adenovirus death protein (ADP) increases the oncolytic efficacy, and hGM-CSF inserts the late gene L3 to stimulate the body’s immunity.
02. Immune stimulating genes that improve therapeutic effects
In order to enhance the therapeutic effect of oncolytic adenovirus, a variety of strategies can be optimized to enhance the effect of oncolytic adenovirus.
Common strategies include: enhancing virus-induced cell apoptosis, virus transmission, expression of specific genes, and stimulating immune response.
Commonly used free immunostimulating factors have: TNF-α, IFN-α , IL-2, IL-12, CD40L, OX40L GM-CSF, and the like.
Delivery optimization of oncolytic adenovirus
In order to ensure the effective anti-tumor effect of oncolytic adenovirus, the optimization and improvement of delivery methods are mainly improved from three aspects.
01. Modified adenovirus phagocytic
The process of adenovirus infecting cells starts from the adhesion of the scalp region of adenovirus cilia to specific receptors on the cell surface.
Human adenoviruses mainly share a receptor with Coxsackie B virus, so this receptor is Called the Coxsackie/Adenovirus receptor or CAR, the tripeptide RGD on the surface of the penton at the base of the virus cilia binds to the integrin on the cell surface to internalize the adenovirus into the cell through endocytosis.
Integrin is uniformly expressed in different tissues, but the expression of CAR is different, which affects the infectivity of adenovirus to a large extent.
For example, by integrating the RGD motif into the HI loop of the fiber globular domain or the hexon protein, the infection of cells with low CAR expression can be enhanced.
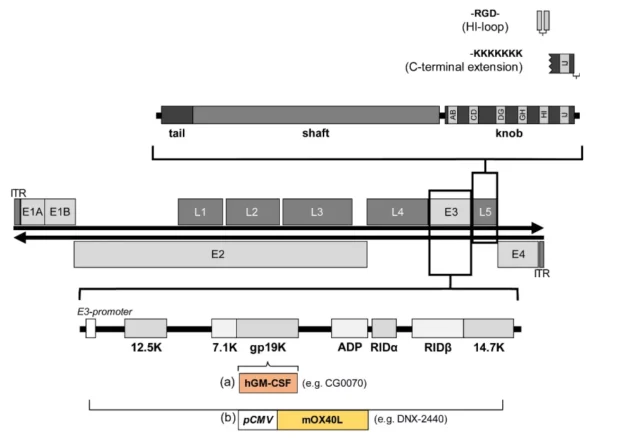
02. Transforming natural tendencies
Adenovirus cell entry receptors are commonly expressed in various tissues of mammals, by changing the RGD motif in the penton base, adding mutations or missing proteins in the fiber, or by combining two different adenovirus types.
A chimera is formed between them to eliminate the tropism of natural viruses to ensure tumor-targeted therapeutic effects.
03. Modified virus delivery
First, the oncolytic adenovirus is used to infect nerve or mesenchymal stem cells, which is used as a transport carrier cell, and then delivered into the patient’s body by intratumoral or systemic administration to deliver the virus to the lesion.
Clinical trials of oncolytic adenovirus
Of the 59 trials at different stages from 2005 to the present, 17 have been completed and 38 are in progress. Table 1-5 provides a detailed overview of the clinical trials collected on the website.
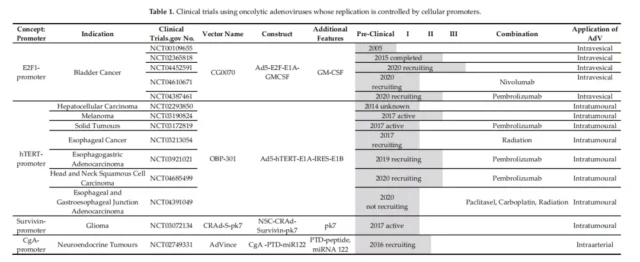
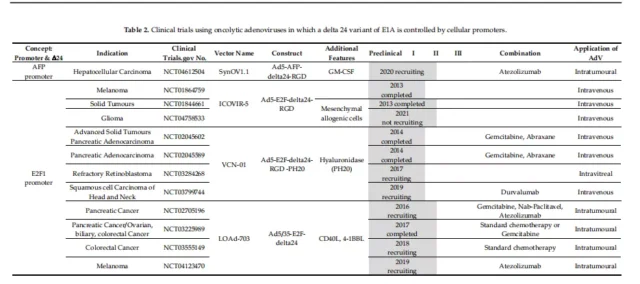
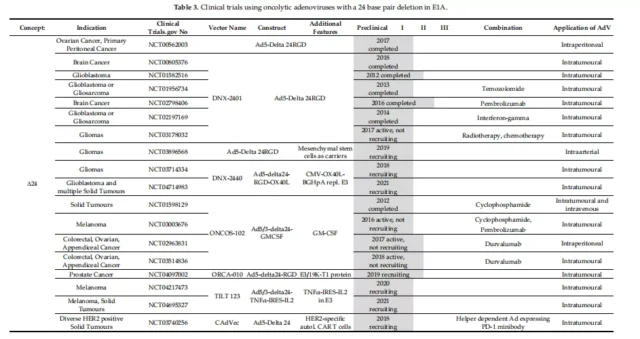
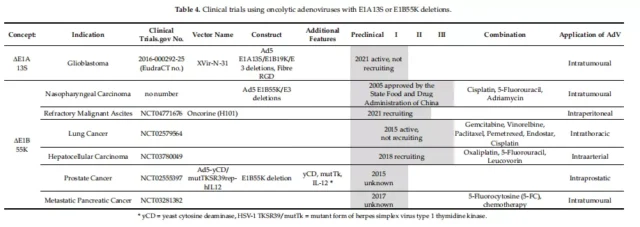
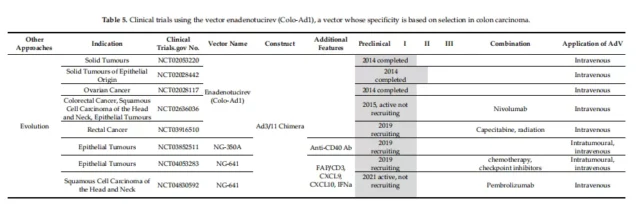
01. Specific clinical vectors for initiating E1A
Oncolytic adenovirus OBP-301, in which the hTERT promoter regulates both E1A and E1B, and they are connected by an IRES element.
The vectors used by the E2F-1 promoter for E1A transcription are Ar6pAE2fE3F and Ar6pAE2fF (with and without the virus E3 region, respectively).
Further optimization on the basis of these vectors, the oncolytic adenovirus vector CG0070 with the GMCSF gene inserted into the E3 region, etc. (Clinical Phase III).
02. E1A-Delta-24 clinical carrier with E1A deletion
The first AdDelta-24 and its derivative DNX-2401 (Delta-24RGD) to be tested in clinical trials for the treatment of gliomas, and four phase I trials have been completed.
03. Specific start of clinical carrier E1A-Delta-24
In a phase I trial of patients with melanoma (NCT01864759), ICOVIR-5 (Ad5-E2F-Delta-24-RGD) was administered systemically intravenously and was well tolerated.
VCN-01 (Ad5-E2F-Delta-24-RGD-PH20) expresses hyaluronidase (PH20), which can enhance the intratumoral transmission of the virus, and its administration is well tolerated. Cell tumors exhibit anti-tumor activity.
04. E1B 55K deletion clinical carrier
ONYX-015 (dl1520) is the first oncolytic adenovirus used in clinical trials for the treatment of head and neck cancer. Oncorine (H101) was approved by the State Food and Drug Administration (SDFA) of China as the world’s first in 2005 Commercial oncolytic virus, used in combination with chemotherapy to treat squamous cell carcinoma of the head and neck (SCCHN).
Outlook
Oncolytic adenoviral vectors achieve systemic therapy through three aspects: tissue specificity, replication and lysis capabilities, and triggering the immune system.
Its therapy has great potential in clinical applications, but it is still in the exploratory stage.
Viruses activate the immune system and introduce transgenes or combine them with immune checkpoint inhibitors to provide new directions and great possibilities for new treatment strategies for adenovirus cancer.
The review of oncolytic adenovirus therapeutic vectors
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



