EGFR-MET bispecific antibody: treatment of lung cancer
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
EGFR-MET bispecific antibody: treatment of lung cancer
EGFR-MET bispecific antibody: treatment of lung cancer. Johnson & Johnson amivantamab applied for listing in the EU: treatment of lung cancer with EGFR exon 20 insertion mutation (NSCLC)!
Johnson & Johnson ’s Janssen Pharmaceuticals recently announced that it has submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) to seek approval for amivantamab (JNJ-61186372, JNJ-6372) for the treatment of patients receiving platinum Patients with metastatic non-small cell lung cancer (NSCLC) with an insertion mutation in exon 20 of the epidermal growth factor receptor (EGFR) gene after the failure of chemotherapy. Earlier this month, Janssen Pharmaceuticals also submitted a Biologics License Application (BLA) for the above-mentioned indications of amivantamab to the US Food and Drug Administration (FDA).
It is worth mentioning that this marks the first regulatory application filed by the European Union and the United States for the treatment of NSCLC patients with EGFR exon 20 insertion mutations. If approved, amivantamab will become the first therapy specifically targeting EGFR exon 20 insertion mutation NSCLC.
Amivantamab is a fully human EGFR-mesenchymal epidermal transformation factor (MET) bispecific antibody under development. It has immune cell targeting activity and targets tumors with activated and drug-resistant EGFR and MET mutations and amplifications. The production and development of amivantamab follow the license agreement signed by Janssen Biotechnology and Genmab to use the DuoBody technology platform.
Both amivantamab BLA and MAA are based on the results of the Phase I CHRYSALIS study (NCT02609776). The data shows that in patients with advanced NSCLC who carry EGFR exon 20 insertion mutations, amivantamab treatment shows a lasting remission:
(1) Among all evaluable patients, the overall response rate (ORR) is 36%, the median duration of response (DOR) is 10 months, and the clinical benefit rate (≥ partial response [PR] + disease stability ≥ 12 weeks) 67%;
(2) Among evaluable patients who had previously received platinum-containing chemotherapy, the ORR was 41%, the median DOR was 7 months, and the clinical benefit rate was 72%.
Based on the ORR and DOR data of the CHRYSALIS study, in March this year, the US FDA granted amivantamab breakthrough drug designation (BTD) for the treatment of patients with metastatic NSCLC who have undergone platinum-containing chemotherapy and have insertion mutations in EGFR exon 20 .
Dr. Peter Lebowitz, MD, Head of Oncology Global Therapy of Janssen Research and Development Company, said: “The submission of the EU MAA is an important milestone in our commitment to developing innovative targeted therapies for lung cancer patients. This is our step towards this goal This is an important step to improve the prognosis of NSCLC patients with EGFR exon 20 insertion mutations. There is currently no approved targeted therapy for such patients.”
Globally, lung cancer is the most common type of cancer, and non-small cell lung cancer (NSCLC) accounts for 80-85% of all lung cancers. The main subtypes of NSCLC are adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. The most common driver mutation in NSCLC is a change in the EGFR gene, which is a receptor tyrosine kinase that helps cells grow and divide.
EGFR mutations are present in 10%-15% of NSCLC patients and 40%-50% of Asian NSCLC adenocarcinoma patients. EGFR exon 20 insertion mutation is a unique subgroup of lung adenocarcinoma, accounting for at least 9% of all EGFR mutations. Currently, the 5-year survival rate for patients with metastatic NSCLC is only 6%.
NSCLC patients with insertion mutations in EGFR exon 20 are usually insensitive to the approved GFR receptor tyrosine kinase inhibitor (TKI) treatment, and are compared with the more common EGFR mutations (exon 19 deletion/L858R substitution) ), the prognosis is worse.
At present, for lung cancer patients with insertion mutations in EGFR exon 20, the estimated median overall survival (OS) is 16 months. The standard clinical care plan is conventional cytotoxic chemotherapy, and there is no approved target therapy.
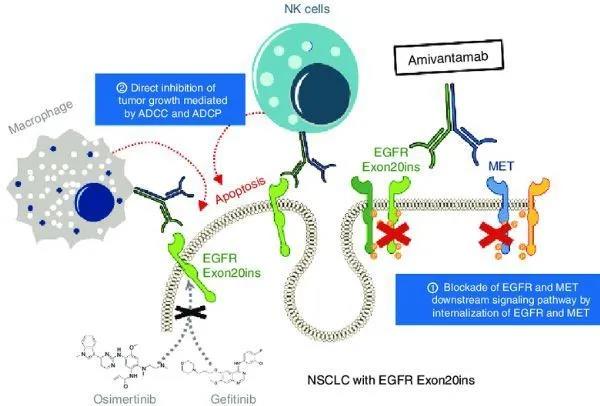
CHRYSALIS is a first-time human, open-label, multi-center phase I study that is evaluating the safety, pharmacokinetics, and pharmacokinetics of amivantamab as a monotherapy and in combination with the new third-generation EGFR-TKI drug lazertinib in the treatment of adult patients with advanced NSCLC Efficacy.
In this study, 50 NSCLC patients with EGFR exon 20 insertion mutations received the recommended phase II dose (RP2D: 1050 mg, 1400 mg for patients with body weight ≥ 80 kg) amivantamab treatment. Of these 50 patients, 39 patients can be assessed for remission and have received ≥2 disease assessments, of which 29 have previously received platinum-containing chemotherapy. Thirteen different EGFR exon 20 insertion mutations were found in 39 patients.
The data showed that among all evaluable patients, the observed overall response rate (ORR) was 36% (95% CI: 21-53); among patients who had previously received platinum-containing chemotherapy, the observed ORR was 41% ( 95%CI: 24-61). In addition, in all 14 remission patients, the median duration of remission (DoR) was 10 months; among remission patients who had previously received platinum-containing chemotherapy, the median DOR was 7 months.
In all patients, the median progression-free survival (PFS) was 8.3 months (95%CI: 3.0–14.8); among patients who had previously received platinum-containing chemotherapy, the median PFS was 8.6 months (95%CI :3.7–14.8).
In all patients, the clinical benefit rate (≥partial remission [PR] or stable disease≥11 weeks) was 67% (95%CI: 50-81); among patients who had previously received platinum-containing chemotherapy, the clinical benefit rate 72% (95%CI: 53-87).
Remission was observed in patients who had previously received treatment and previously received platinum-containing chemotherapy. Tumor response is most common in the first disease assessment after starting treatment.
In the study, the most common adverse events (AE) of all grades were skin rash, infusion-related reactions (IRR) and paronychia. IRR mainly occurs during the first infusion and does not prevent subsequent infusion therapy. There was no report of rash ≥3 grade, and 1 patient had grade 3 diarrhea (6% of patients had diarrhea of any grade).
6% of patients had treatment-related grade 3 AEs, including hyperamylaseemia, hypokalemia, elevated lipase, and shoulder/chest pain. 6% of patients reported serious adverse events related to treatment, such as cellulitis, interstitial lung disease, shoulder/chest pain.
(source:internet, reference only)
Disclaimer of medicaltrend.org



