Death of non-small cell lung cancer reduced 42% by Tecentriq Combination
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Death of non-small cell lung cancer reduced 42% by Tecentriq Combination
Death of non-small cell lung cancer reduced 42% by Tecentriq Combination. With the new therapies approved by the FDA, the dual-drug combination remission rate has almost doubled, and the risk of death has also been greatly reduced.
Lung cancer is the leading cause of cancer deaths worldwide. About 1.6 million people die from lung cancer every year. Among them, approximately 85% of lung cancer patients have a histological subtype of non-small cell lung cancer (NSCLC).
From the initial cytotoxic chemotherapy to targeted therapy, to the current immunotherapy, the treatment of non-small cell lung cancer has made significant progress.
On January 5, 2021, the U.S. Food and Drug Administration (FDA) granted tiragolumab breakthrough therapy qualification. The therapy is: the combined application of tiragolumab and atelizumab (Tecentriq), the first-line treatment of patients with metastatic non-small cell lung cancer (including adenocarcinoma and squamous cell carcinoma).
Tiragolumab is a monoclonal antibody that can target and bind to TIGIT (protein receptor on immune cells) to play a role in immune amplification; Tecentriq is an anti-PD-L1 antibody. The combination of the two, while targeting the immunosuppressive receptors TIGIT and PD-L1, can enhance anti-tumor activity by amplifying the immune response.
In fact, as early as the American Society of Clinical Oncology (ASCO) annual meeting in 2020, Roche declared that it had high hopes for the combination of tiragolumab+Tecentriq, hoping to become the best choice for the first-line treatment of metastatic non-small cell lung cancer.
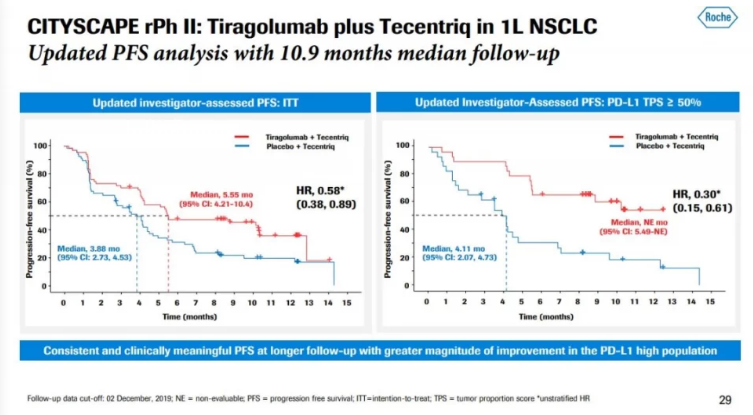
The reason for saying this is because they did a study: the study enrolled 135 patients with EGFR-, ALK-, PD-L1 positive non-small cell lung cancer and evaluated the first-line combination of tiragolumab+Tecentriq and Tecentriq alone. The effectiveness and safety of the treatment of metastatic non-small cell lung cancer.
The research results are as follows:
01. The total remission rate achieved with Tecentriq alone was 21%, and the total remission rate with the combination of tiragolumab+Tecentriq was 37%. At the same time, the combination therapy reduced the risk of disease deterioration or death (progression-free survival PFS) by 42%.
02. Among the people with high levels of PD-L1 (tumor ratio score TPS≥50%), the total response rate achieved by Tecentriq alone was 24%, and the total response rate by the combination of tiragolumab+Tecentriq was 66%.
03. In terms of safety, tiragolumab + Tecentriq is well tolerated. Compared with Tecentriq alone, the incidence of all grade 3 or more cause adverse events is similar (48% vs. 44%).
It can be seen that the combination of tiragolumab+Tecentriq is more effective in treating metastatic non-small cell lung cancer!
Write at the end
We will continue to pay attention to this promising clinical treatment and look forward to it providing an effective treatment plan for lung cancer patients
(source:internet, reference only)
Disclaimer of medicaltrend.org