Capillary electrophoresis: Application in protein drug analysis
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Capillary electrophoresis: Application in protein drug analysis
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- What is the difference between Atorvastatin and Rosuvastatin?
- How long can the patient live after heart stent surgery?
Capillary electrophoresis: Application in protein drug analysis.
Capillary electrophoresis (CE) has become more and more favored by major biopharmaceutical companies and attention by regulatory agencies.
Most biopharmaceutical companies in the world have used capillary electrophoresis systems for protein drug development and quality control analysis.
1. The development of capillary electrophoresis technology in the development and quality control of protein drugs
With the rise of protein drug development around the world, capillary electrophoresis (CE), as an emerging R&D and quality control analysis technology, has become more and more favored by major biopharmaceutical companies and attention by regulatory agencies.
Most biopharmaceutical companies in the world have used capillary electrophoresis systems for protein drug development and quality control analysis.
From medium optimization, clone screening, formulation stability research and purification process monitoring, to protein characterization, related impurity detection, protein structure identification and protein drug product quality control, capillary electrophoresis is required in all aspects of protein drugs.
For example, protein purity determination has been transformed from SDS-PAGE to sodium dodecyl sulfate-capillary gel electrophoresis (CE-SDS) method; protein isoelectric point determination, capillary isoelectric focusing (CIEF) is better than traditional gel strip method More accurate; Capillary electrophoresis is one of the high-resolution analysis methods for the characterization of glycosylation heterogeneity of glycoprotein drugs.
In the pharmacopoeias of various countries, the detection methods of capillary electrophoresis technology for protein drugs are also continuously enriched and developed.
The earliest detection method for protein drugs in the pharmacopoeia is the determination of the sugar isomer of erythropoietin (EPO).
The isomers of glycoproteins have small differences, and it is difficult to separate and quantify multiple isomers in EPO with ordinary analysis methods.
The European Pharmacopoeia and the United States Pharmacopoeia established the capillary electrophoresis method as the standard for the analysis of EPO isomers to solve the separation and quantification of various glycosylated isomers in EPO products.
In addition, capillary electrophoresis is also used in the detection standards for related impurities of growth hormone.
For the analysis of monoclonal antibody drugs, in 2006, more than a dozen laboratories including Wyeth, Amgen, Gene Technology, Eli Lilly, Pfizer, Johnson & Johnson, and Health Canada conducted “CE-SDS method for mAb drug purity analysis” A joint verification was carried out [1].
They researched and inspected the stability, reliability and accuracy of the method.
The results of the study show that the CE-SDS method is more suitable for the characterization and quality control of monoclonal antibody drugs than the traditional SDS-PAGE, and the stability and reliability of the results far exceed SDS-PAGE. It is recommended that all biopharmaceutical companies use CE-SDS instead of the original SDS-PAGE as a platform for R&D and quality analysis.
Subsequently, the above-mentioned biopharmaceutical companies and institutions targeted the “CIEF method for the determination of the isoelectric point of monoclonal antibodies and the analysis of charge heterogeneity [2]” and “the CZE method for rapid analysis of the charge heterogeneity of monoclonal antibodies [3]” , “Capillary electrophoresis technology for glycan analysis in monoclonal antibodies [4]” was jointly verified by multiple laboratories, and the results showed the advantages and feasibility of CE technology for quality control of monoclonal antibodies.
The United States Pharmacopeia published in 2013 the purity detection, isoelectric point/charge heterogeneity analysis, and sugar analysis using capillary electrophoresis methods for monoclonal antibody drugs such as rituximab and troutozol.
The CE technology was added to the third part of the Chinese Pharmacopoeia 2015 edition, clarifying that CE is an important method in the analysis of monoclonal antibody drug size variants, charge variants, identification and consistency and glycosylation modification.
With the rapid development of CE technology in the biopharmaceutical field and the continuous listing of new protein drugs, more CE methods will appear in the pharmacopoeias of various countries.
2. Application of capillary electrophoresis technology in the analysis of monoclonal antibody drugs
2.1. Analysis of purity and size heterogeneity of monoclonal antibody drugs
The SDS-PAGE method for purity analysis of monoclonal antibody drugs, in terms of resolution, quantitative accuracy and automation, can no longer meet the requirements of biopharmaceutical research and development and quality control.
The CE-SDS method is based on the differential separation of protein molecular weights. It is used for the purity analysis of reduced and non-reduced monoclonal antibody drugs.
It eliminates complicated manual operations, makes quantification more accurate, has higher resolution, and can be used for non-reduction in the reduction mode.
The glycosylated heavy chain is separated and accurately quantified.
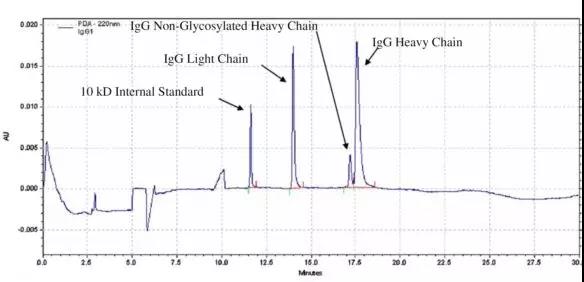
Figure 1. CE-SDS purity analysis of reduced monoclonal antibody drugs [1]
Different capillary lengths can be used to realize purity analysis in high-resolution mode and fast mode. The CE-SDS method in high resolution mode provides the highest resolution, and the CE-SDS method in fast mode provides shorter washing and separation times, which improves the throughput of analysis.
CE-SDS combined with laser-induced fluorescence detector (CE-SDS-LIF), the protein is labeled with 5-Tarma or FQ dye, which can obtain higher sensitivity and can detect impurities with a content of 0.01%. In addition, the use of the LIF detector can minimize baseline fluctuations and make integration and quantification more accurate.
2.2 Determination of isoelectric point of monoclonal antibody drugs and analysis of charge heterogeneity
The structure of monoclonal antibody drugs will undergo post-translational modifications such as glycosylation, deamidation, isomerization, oxidation, etc., which will change the surface charge of the protein and cause the charge heterogeneity of the monoclonal antibody.
Each variant has a different isoelectric point. Capillary isoelectric focusing technology (cIEF) based on isoelectric point separation can separate and quantify the variants of monoclonal antibody drugs with high resolution, and can separate 0.03 variants with pI difference.
Methods The isoelectric point Marker was used to make a calibration curve to accurately determine the isoelectric point of the variant. It is an important method for the determination of the isoelectric point of monoclonal antibody drugs and the analysis of charge heterogeneity.
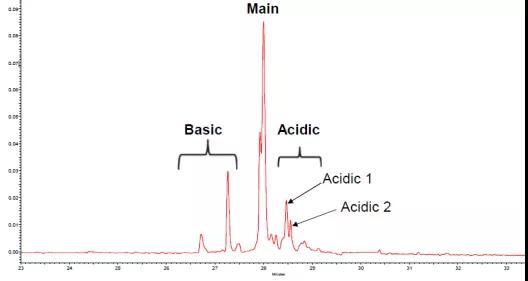
Figure 2. Analysis of isoelectric point and charge heterogeneity of monoclonal antibody drugs by CIEF method [5]
For protein samples with different pI ranges, high-resolution analysis can be achieved by selecting appropriate ampholytes.
For example, for most monoclonal antibodies whose pI value is between 7-10, amphoteric electrolytes with a pH range of 3-10 can be used; for protein samples with a pI in the range of 5-7, a narrow range of pH 5-8 can be used. Electrolyte; For acidic proteins with pI less than 5, reverse focusing and migration modes can be used to achieve better analysis.
2.3 CZE method for rapid analysis of charge heterogeneity of monoclonal antibody drugs
Capillary zone electrophoresis (CZE) performs separation based on the charge/volume ratio of the analyte.
It is the simplest and fastest mode in capillary electrophoresis technology. Because the molecular volume of each variant of the monoclonal antibody drug is almost the same, in the CZE separation mode, the separation of the charge variant depends on the difference in surface charge, which is consistent with the separation of the variant in the CIEF mode.
Therefore, CZE has become a platform method for rapid charge heterogeneity analysis and is used by the biopharmaceutical industry. In addition, due to the simple and rapid characteristics of the CZE method, it has also been used in the identification and analysis of monoclonal antibodies.
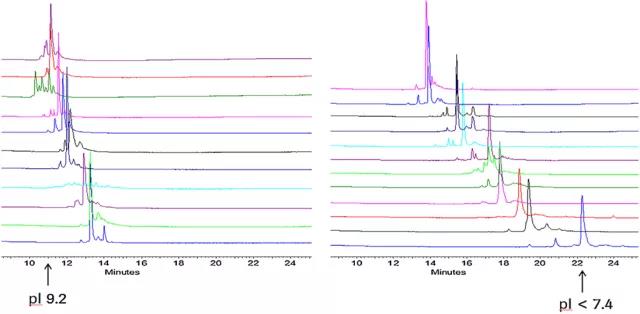 Figure 3. Analysis of charge heterogeneity of 23 monoclonal antibody drugs by the same CZE method [3]
Figure 3. Analysis of charge heterogeneity of 23 monoclonal antibody drugs by the same CZE method [3]
2.4 Analysis of glycosylation heterogeneity of monoclonal antibody drugs
In glycoprotein drugs such as monoclonal antibodies, the type and order of glycosyl groups can cause glycosyl heterogeneity. The glycosylation modification of monoclonal antibody drugs has a great impact on its safety and efficacy.
Therefore, the quality control of glycosyl heterogeneity is very important. The procedure of capillary electrophoresis for glycosyl heterogeneity analysis includes the release of glycosyls in glycoproteins, the labeling of glycosyls and capillary electrophoresis separation.
Magnetic bead-assisted glycosyl release and labeling enables the pretreatment to be completed within 1 hour, speeding up the pretreatment time. Using APTS as a fluorescent label can not only increase the separation efficiency by increasing the charge, but also achieve highly sensitive glycosyl analysis through LIF detection.
The advantages of capillary electrophoresis for glycosyl analysis are high resolution and fast speed. Not only can the difference of a sugar group be distinguished, but the sugar group isomers of the same molecular weight can also be separated, and the entire separation process can be completed within 5-20 minutes.
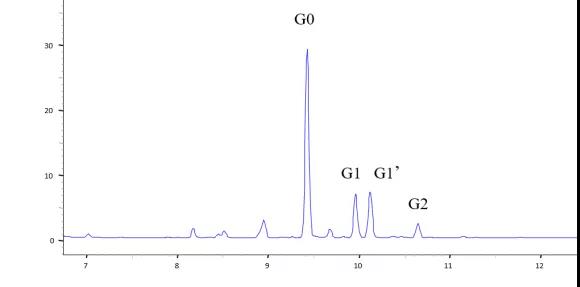
Figure 4. The electrophoresis diagram of the CE-LIF method for glycan analysis of monoclonal antibodies
3. Application of capillary electrophoresis technology in the analysis of recombinant protein drugs
Recombinant human erythropoietin (rhEPO) is a highly glycosylated protein drug. The heterogeneity of glycosylation leads to the existence of multiple variants. The CZE method can be used to separate and quantify EPO variants. This method has become the standard method for EPO variant analysis in the European Pharmacopoeia.
In addition, the CIEF method can also achieve high-resolution separation of various variants in EPO, not only can obtain the same number of variants and quantitative information as the CZE method, but also provide accurate isoelectric point values for each variant.
In comparing EPO products from different sources with reference products, the isoelectric point can be used to identify variants.
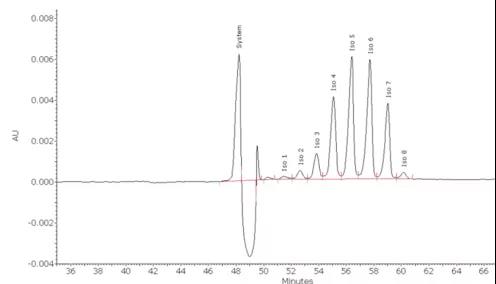 Figure 5. Analysis of EPO variants by CZE method
Figure 5. Analysis of EPO variants by CZE method
In the purity and heterogeneity analysis of recombinant human growth hormone (rhGH), the CZE method has high resolution and accurate quantification, and has also been adopted by the European Pharmacopoeia.
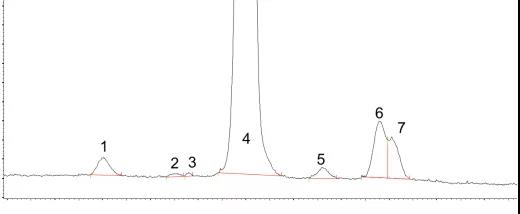
Figure 6 Analysis of charge heterogeneity of rhGH by CZE method
4 Summary
Today, with the vigorous development of protein drugs, capillary electrophoresis technology has played an indispensable role in the process of protein drug development and quality control due to its advantages of high resolution and multiple modes. It has been used by more and more companies and regulatory agencies.
Approved for use in the analysis of protein drug purity, isoelectric point, charge heterogeneity, and glycosyl. With the continuous development of biological products such as protein drugs, cell/gene therapy, and new vaccines, capillary electrophoresis technology will have greater application space.
In the analysis of proteins, nucleic acids and virus particles, it will give full play to its advantages and improve biological products. Quality control standards.
Capillary electrophoresis: Application in protein drug analysis
(source:internet, reference only)
Disclaimer of medicaltrend.org



