Progress and principle of combined immunotherapy for gastroesophageal cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Progress and principle of combined immunotherapy for gastroesophageal cancer
Progress and principle of combined immunotherapy for gastroesophageal cancer. This article summarizes and reviews the current progress of these novel combination therapies and explains their basic principles.
Previous studies have shown that anti-angiogenic agents and immune checkpoint inhibitors are effective for patients with advanced gastric cancer (GC) and gastroesophageal junction adenocarcinoma (GEJC). Ramucirumab is an anti-VEGFR2 antibody that has shown efficacy in GC, but its benefits are limited, partly due to MET-mediated resistance. Other TKIs that target VEGF, such as regorafenib and cabozantinib, have a broad spectrum of multikinase inhibition, and have also shown moderate single-agent activity in early trials.
For immune checkpoint inhibitors, pembrolizumab (anti-PD-1) as a third-line therapy for PD-L1 expressing GC and GEJC populations has survival benefits and has been approved by the FDA for this indication. Preclinical data has proved the extensive tumor microenvironmental immunomodulatory effects of anti-angiogenic agents, which supports the clinical research principle of dual blockade of VEGF and immune checkpoints.
In addition, the FDA has approved the combined use of anti-VEGF / VEGFR and anti-PD-1 / PD-L1 drugs in hepatocellular carcinoma and renal cell carcinoma. In several phase I/II clinical trials, the dual-blocking combination therapy of anti-angiogenic agents and immune checkpoint inhibitors has demonstrated potential clinical activity in patients with refractory GC/GEJC. This review summarizes and reviews the current progress of these novel combination therapies and explains their basic principles.

Overview of the main content
- Overview of the background of gastroesophageal cancer treatment and IO combined treatment
- Clinical progress of anti-angiogenesis and IO monotherapy for gastroesophageal cancer
- Principles of angiogenesis and tumor immune microenvironment
- Progress in the combination therapy of anti-angiogenesis and IO in gastroesophageal cancer
- Hallenges and future directions
- Conclusion
Overview of the background of gastroesophageal cancer treatment and IO combined treatment
Although immune checkpoint inhibitors (IO) show durable responses and prolonged survival for multiple tumor types, the proportion of patients with metastatic cancer who benefit from IO is still limited, including gastric cancer and gastroesophageal cancer (GC/GEJC). It is worth noting that, considering the similar underlying pathogenesis and genomic changes between GC and GEJC, they have been regarded as a clinical entity and managed as a clinical entity, so they will be discussed here as a cancer type .
In locally advanced metastatic GC/GEJC, although single-agent IO therapy has been used in the subgroup of patients with high mismatch repair/microsatellite instability (dMMR/MSI-H) and in third-line patients with high PD-L1 expression Shows benefits, but most of the remaining patients have limited benefits. Researchers have been working to develop new strategies to extend clinical benefits to non-responders, and one of these strategies is to combine IO with other systemic treatments.
Part of the primary resistance to IO therapy is due to the immunosuppressive properties of the tumor microenvironment (TME). Immunosuppressive TME is characterized by increased infiltration of immunosuppressive cells, including regulatory T cells (Treg), myeloid-derived suppressor cells (MDSC), tumor-associated macrophages (TAM), especially the tumor-promoting M2 phenotype. These features reduce the activity of immune effector cells (such as cytotoxic T cells and helper T cells) through a variety of mechanisms (such as the production of immunosuppressive cytokines and chemokines and abnormal metabolic pathways). Although the biological mechanisms leading to immunosuppressive TME are multifaceted and complex, one of the most understood and important mechanisms is the role of neovascularization.
The tumor angiogenesis mediator with the best immunosuppressive effect is vascular endothelial growth factor (VEGF). It is a cytokine induced by local tissue hypoxia and acidosis, which can promote the growth of defective and leaky tumor blood vessels. In addition to the indirect effects of immunosuppression by blocking the tumor infiltration of immune effector cells through its influence on the vasculature, VEGF also has direct local and systemic immunosuppressive effects. For example, VEGF is associated with increased tumor invasion of Treg, MDSC, and M2 macrophages, and anti-VEGF therapy leads to the reversal of these immunosuppressive mechanisms. Therefore, anti-VEGF therapy is related to the “normalization” of TME, and has the potential to reverse the resistance to IO therapy, and is a promising combination therapy partner.
The combination of VEGF and PD-1/PD-L1 axis blockers has shown advantages in a variety of tumors and is becoming a promising combination strategy. A well-known and successful example is the IMBrave150 trial, in which bevacizumab (anti-VEGF-A) and atilizumab (anti-PD-L1) have better overall survival (OS) than sorafenib and sora Fenis is the standard for first-line treatment of hepatocellular carcinoma (HCC) for the treatment of metastases. In addition, the preliminary results of the phase I/II trial of the multi-group COSMIC-312 trial indicated that cabozantinib is a multi-receptor tyrosine kinase (TKI) inhibitor with VEGF inhibitory activity. Anti-combination for metastatic HCC, small cell lung cancer, renal cell carcinoma (RCC) and prostate cancer has extraordinary potential. In this article, we summarize the preclinical evidence supporting the synergy of anti-VEGF and anti-PD-1/PD-L1 in metastatic GC/GEJC, and review the results of ongoing clinical trials evaluating this regimen to prove this Emerging combination therapy is expected to improve the treatment model of metastatic GC/GEJC.
Clinical progress of anti-angiogenesis and IO monotherapy for gastroesophageal cancer
▶ Current status of gastroesophageal antivascular therapy
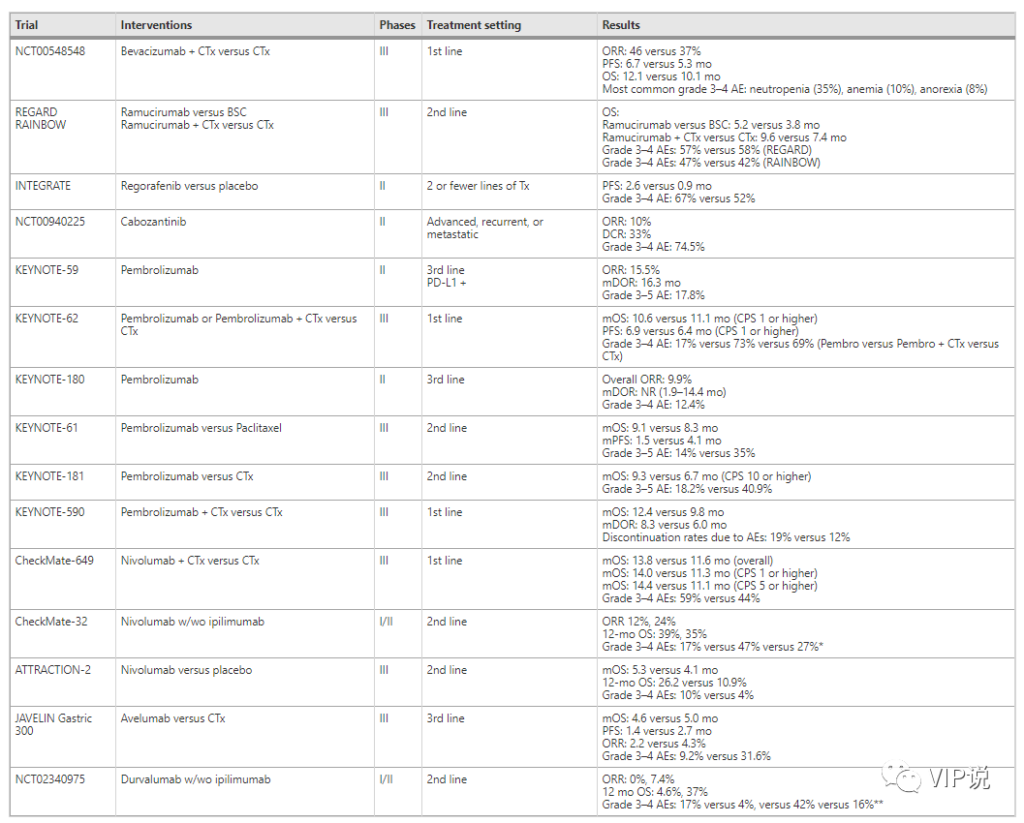
Compared with chemotherapy alone, bevacizumab was tested in the first-line treatment of metastatic chemotherapy in patients with metastatic chemotherapy. The combination showed improvement in remission rate (46% vs 37%) and PFS (6.7 vs 5.3 months). Unfortunately, the benefits of OS are not statistically significant (12.1 vs 10.1 months). Ramucirumab received survival benefits in single-agent therapy and optimal supportive care (5.2 vs 3.8 months) or combined therapy with paclitaxel and paclitaxel alone (9.6 vs 7.4 months).
In addition, regorafenib showed a significant improvement in the median PFS in a phase II randomized placebo-controlled trial (INTEGRATE), which was 2.6 months and 0.9 months, respectively. Cabozantinib showed efficacy and safety in a phase 2 randomized trial (526 patients with 9 tumor types, including 21 GC patients). The primary endpoint ORR at week 12 in the GC group was 10%, and the disease control rate was 33%.
▶ Current status of gastroesophageal IO treatment
The IB KEYNOTE-028 phase clinical trial is the first to study the safety and treatment response of the anti-PD-1 antibody pembrolizumab in patients with unresectable esophageal cancer with PD-L1 expression who have received second-line and above treatment (ORR 30 %, median duration of response (mDOR) 15 months). This study was followed by a phase II clinical trial of KEYNOTE-059, which confirmed the efficacy of pembrolizumab in the PD-L1+ population (ORR 15.5%, mDOR 16.3 months).
The Phase I/II CheckMate-032 trial demonstrated the potential efficacy of nivolumab alone or in combination with Ipilimumab in GEJC patients who have progressed through first-line treatment and above (in advanced GC/medium, N and N+I The ORR was 12%, 24%; the 12-month OS was 39%, 35%). In addition, in the ATTRACTION-2 trial, in non-selected patients with non-relapsed or refractory unresectable GC biomarkers, nivolumab showed a higher median OS (5.3 vs. 4.1 months, P <0.0001) and 12-month OS rate (26.2 vs. 10.9%).
On the other hand, anti-PD-1 drugs have also shown disappointing results, and new strategies must be adopted to overcome primary or secondary resistance to treatment. For example, the phase III KEYNOTE-062 trial failed to prove the superiority of pembrolizumab compared with front-line chemotherapy. Even in the PD-L1> 10% subgroup, pembrolizumab has no advantage over chemotherapy. In addition, the phase II KEYNOTE-180 trial showed that the response rate of pembrolizumab in the third-line treatment of GEJ cancer was disappointing (ORR 5.2%).
Similarly, the phase III KEYNOTE-181 trial failed to show the OS benefit of pembrolizumab over chemotherapy in the first-line treatment of advanced or advanced esophageal cancer. Finally, the phase III KEYNOTE-061 trial failed to show the OS benefit of pembrolizumab over chemotherapy in the second-line treatment in the unselected GC/GEJC population.
Although anti-PD-1 therapy alone has not achieved good results in a frontline environment, the combination of anti-PD-1 therapy and chemotherapy has shown good results in some people. In other words, the phase III KEYNOTE-590 trial (pembrolizumab + chemotherapy vs. chemotherapy) showed a higher median OS (median OS 12.4 vs 9.8 months; HR, 0.73, 95% CI, 0.62-0.86 ) And PFS (median 6.3 vs 5.8 months; HR 0.65; 95% CI), including 73% of esophageal squamous cell carcinoma and 27% of esophageal adenocarcinoma.
It is worth noting that in the subgroup with CPS ≥ 10, OS benefit (median 13.5 vs 9.4 months; HR 0.62; 95% CI, 0.49–0.78) and PFS benefit (median 7.5 vs 5.5 Month; HR 0.51; 95% CI, 0.41-0.65) is greater. Similarly, in the Phase III CheckMate-649 trial, compared with chemotherapy alone, nivolumab plus chemotherapy has a better effect. It is worth noting that this patient population is only composed of GC/GEJC patients, and is rich in a subgroup of patients with PD-L1 CPS>5%, which accounts for >50% of the total study population. Nivolumab combined with chemotherapy showed a superior median OS (HR 0.80, 99.3% CI, 0.68-0.94) in the total population. In summary, these studies show that in selected populations, appropriate combination of IO therapy can overcome treatment resistance.
Principles of angiogenesis and tumor immune microenvironment
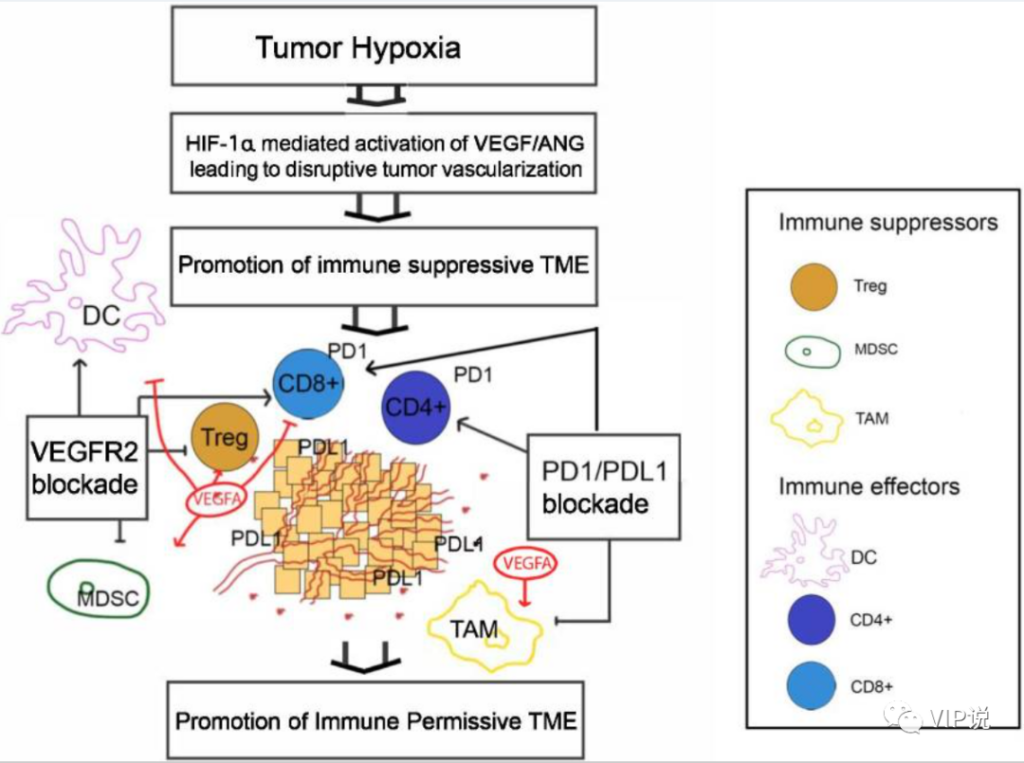
There is ample evidence that the angiogenesis pathway can promote immunosuppressive tumor microenvironment (TME) in many ways, including by directly inhibiting antigen presenting cells and immune effector cells or by enhancing immunosuppressive cells (such as Treg, MDSC and TAM) .
VEGF-driven angiogenesis involves its homologous receptor VEGFR1-3, which is the most important angiogenic factor associated with the immunosuppressive effects of TME in various aspects. For example, the binding of VEGF to the receptors on DCs will inhibit their maturation and antigen presentation, and induce PD-L1 expression on the cell surface. In addition, increased VEGF levels lead to inhibition of cytotoxic T cell trafficking, proliferation and effector functions. In addition, VEGF promotes the expansion of Treg and MDSC and TAM in TME. This angiogenic pathway other than VEGF also contributes to immunosuppression in TME. For example, Ang-2 is a cytokine ligand for the Ang1/Tie2 receptor, which regulates VEGF-mediated angiogenesis. The expression of Ang-2 leads to increased endothelial adhesion of TIE-2 expressed monocytes/macrophages and stimulates their production of immunosuppressive cytokines such as IL-10.
In turn, it has been found that targeting VEGFR using various methods including multi-target VEGF TKIs can promote immune permissible TME by normalizing blood vessel formation and reducing MDSC (CD11b +, Gr +). In addition, VEGF TKI has been described to have immunomodulatory effects on several cancers in vitro and in murine models. For example, cabozantinib appears to drive its effect on Treg through the HGF/c-Met pathway, where this receptor signaling cascade can regulate multiple immune cell functions. In an autoimmune model of the mouse central nervous system, HGF has been shown to induce Treg (CD4 + CD25 + FoxP3) by blocking DC function. Monocytes cultured with HGF differentiate into monocytes and produce soluble factors (such as IL-10), which are known to promote ideal immunosuppressive conditions for tumor development.
The immunomodulatory effect of cabozantinib was mainly studied in in vivo and in vitro tumor models other than GC/GEJC. For example, in murine colorectal cancer tumor cells, cabozantinib leads to increased expression of major histocompatibility complex (MHC) class 1 and cell surface molecule FAS, and promotes the expression of intercellular adhesion molecule 1 and calreticulin , To jointly enhance the recognition ability of immune cells and enhance the sensitivity to T cell-mediated lysis.
In addition, treatment with cabozantinib can enhance peripheral CD8 + T cells and reduce Treg and MDSC in the spleen. In murine tumor models, significant tumor infiltration of CD8 + T cells and Tregs and reduction of tumor infiltration of MDSC and TAM were observed. These results indicate that cabozantinib reduces the immunosuppressive environment of mouse tumors. In a chimeric mouse model of metastatic castration-resistant prostate cancer, when cabozantinib is used as a single drug, it has little effect on the tumor mass, but when used in combination with anti-CTLA-4 or anti-PD-1, A powerful synergistic response mediated by neutralizing MDSC (CD11b +, Gr1 +) was observed.
It was also found that PI3K signaling was blocked by cabozantinib, which impaired the cytokine release of prostate cancer cells. These in turn increase the expression of MDSC genes responsible for tumor suppressor activity. The negative regulation of GR1 + MDSCs is related to the increase of CD8 + T cell tumor infiltration in prostate tumors, which proves the antagonistic activity of Gr1 + MDSCs on T cell CD8 + population.
In addition to cabozantinib, other VEGF TKIs have shown immunomodulatory effects in in vitro and in vivo studies, further supporting the potential role of VEGF TKIs in combined IO therapy. For example, lenvatinib is associated with a decrease in TAM and an increase in tumor infiltration of interferon gamma-producing CD8+ T cells and memory T cells in various tumor models. In addition, regorafenib is also associated with the decrease of TAM infiltration and the increase of M1 macrophages in the colorectal cancer model.
Progress in the combined therapy of anti-angiogenesis and IO in gastroesophageal cancer
▶ Combination of anti-PD-1/PD-L1 and VEGFR2 monoclonal antibodies
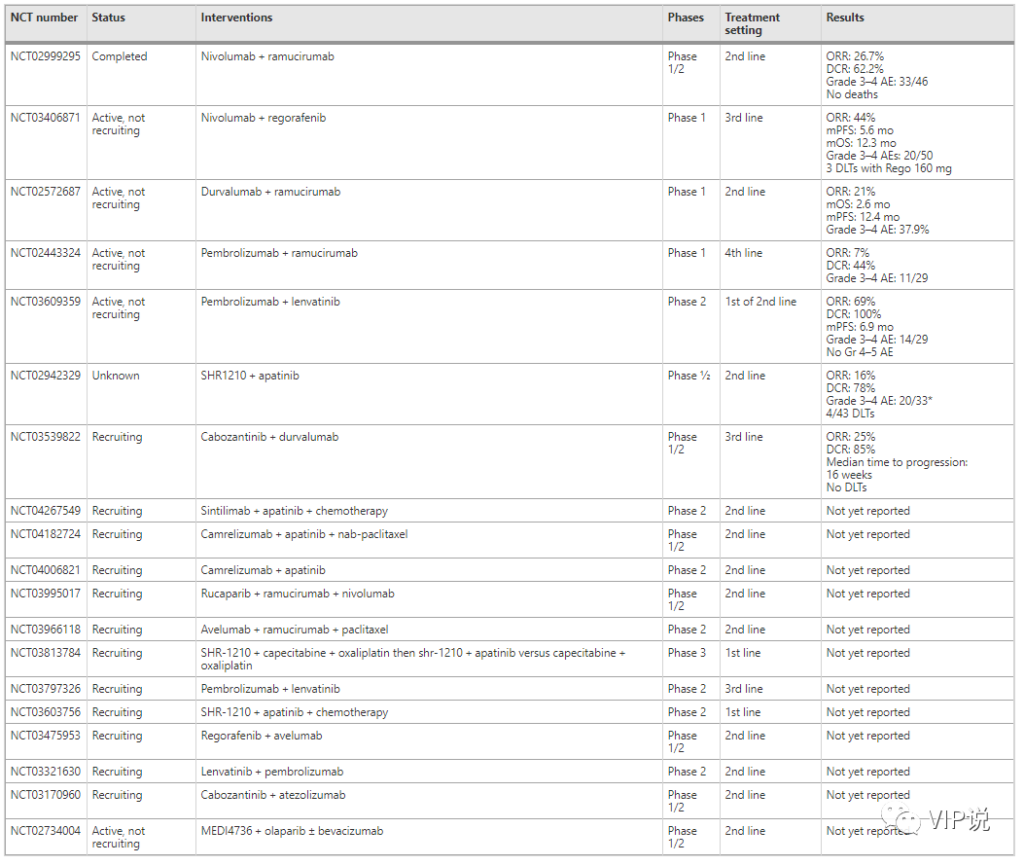
Recent data show that in GC/GEJC patients, ramucirumumab + nivolizumab, ramucirumumab + pembrolizumab and ramucirumumab + duvalizumab Resistance order encouraging activity.
In the phase I/II NivoRam study, ramucirumab and nivolumab were evaluated in the second-line treatment of patients with advanced gastric cancer. The primary endpoints were dose limiting toxicity (DLT) and 6-month PFS. Using the CPS score to assess PD-L1 expression in patients, 44% of patients were determined to have a PD-L1+ cut-off value of 1 or higher. Six patients were evaluated in the first phase, and then another 40 patients were added in the second phase. ORR was 26.7%, and disease control rate (DCR) was 62.2%. No dose limiting toxicity was observed in Phase I. The 6-month PFS was 37.4% (90% confidential interval: 25.7-49.2%), reaching the primary endpoint of the second phase.
Recently, in a multi-cohort Ib trial, in a multi-cohort Ib trial of 3 tumor types including previously treated GC/GEJC (n = 41), the combination of ramucirumab and pembrolizumab Similar combinations of resistance were studied. The primary endpoint is DLT and the incidence of adverse events. Evaluate the tumor’s PD-L1 TPS, MMR/MSI and HER2 status. The combination showed controllable safety with an ORR of 7% and a DCR of 44%. In addition, the combination of ramucirumab+duvalizumab was evaluated in a single-arm Phase Ia trial, which included three cancer types, including GC/GEJC (n=29). This protocol showed promising tumor response (ORR 21%) and survival (OS 2.6 months; PFS 12.4 months).
▶ Combination of anti-PD-1/PD-L1 and VEGFR multi-TKI
The combination of VEGFRTKI plus checkpoint inhibitor has been explored in a single-arm study, and has shown potential efficacy in various tumor types including GC/GEJC.
A related example is regorafenib and navuliyuumab in chemotherapy-refractory gastric cancer, which has an ORR of 44%, compared with 3% for regorafenib alone in the INTEGRATE trial. In addition, the median PFS and OS were 5.6 months and 12.3 months, respectively. The incidence of treatment-related grade 3 or higher adverse events in the entire population was 40%.
Recently, lenvatinib+pembrolizumab has been used in patients with gastric and gastroesophageal adenocarcinoma refractory to chemotherapy and not receiving chemotherapy (14 cases did not receive chemotherapy, 15 cases were chemotherapy refractory), the primary endpoint ORR is 69% and DCR is 100%. The median PFS was 6.9 months. In this study, the MMR/MSI, HER2, EBV, PD-L1 CPS and TMB status of tumor specimens were evaluated. The combination regimen showed good safety, and no grade 4 or grade 5 treatment-related adverse events and grade 3 events occurred in 48% of patients. The most common grade 3 adverse events related to treatment were hypertension, proteinuria, and thrombocytopenia.
The combination of apatinib (VEGFR2 inhibitor) and SHR-1210 (anti-PD-1 antibody) has also been evaluated in a phase I/I multi-cohort trial with patients with advanced gastric cancer and hepatocellular carcinoma. The primary endpoint of the trial is overall survival at 6 months and 12 months. The mid-term results showed that among patients receiving the recommended phase II dose, the incidence of DLT in the total population was 26.7% (4/43), moderate 3-4 adverse events were 60% (20/33), and overall toxicity medium. The confirmed objective response in the GC/GEJC population was 16% (4/25) and 78% in DCR.
The I/II CAMILLA trial tested the treatment of cabozantinib plus duvalimab, mainly for patients with advanced gastric and esophageal adenocarcinoma. The main result of this study is the maximum tolerated dose, which is defined as the highest dose studied. The highest dose observed is DLT and ORR less than 33%. This research is ongoing, but the results of the first phase are encouraging. When the dose of cabozantinib was increased from 20 mg to 60 mg, there was no dose limiting toxicity. Of the 20 patients treated with the composition, 5 (25%) had partial response (PR), while 17 of 20 patients (85%) had DCR (partial response + stable disease). The median time to disease progression (PD) is 16 weeks (range 8–40+).
Challenges and future directions
Although both VEGFR inhibitors and PD-1/PD-L1 inhibitors have been shown to be active as single drugs for the treatment of GC and GEJC, the combination of VEGFR plus PD-1/PD-L1 targeting can lead to benefits and predict treatment response The mechanism needs further study. So far, PD-L1 expression, high TMB and MSI-high status are correlated with PD-1/PD-L1 monotherapy treatment response and survival rate.
According to the CheckMate-649 trial, KEYNOTE-590 and other methods, the expression of PD-L1 and MSI status using the CPS score have been confirmed as predictors of the population’s response to PD-1 plus chemotherapy combinations. Ongoing and future trials of VEGFR plus PD-1/PD-L1 inhibitors should test the predictive value of those similar markers. Exploring other predictive and prognostic markers will add important data to support the field of personalized medicine in immuno-oncology.
In the context of immunity + anti-angiogenesis, there are still challenges in the interpretation and evaluation of the predictive value of biomarkers. Future research on biomarker development is necessary to predict the synergy between IO and VEGF targeting agents. Taking into account the toxicity characteristics of this novel combination, its toxicity is greater than that of any drug alone, so it would be ideal to tailor it to the people who benefit most.
In the context of the combination of immunity and anti-angiogenesis, there are still challenges in the interpretation and evaluation of the predictive value of biomarkers. Future research on biomarker development is necessary to predict the synergy between IO and VEGF targeting agents. Considering the toxicity characteristics of this novel combination, its toxicity is worse than that of any drug alone, so it would be ideal to tailor this strategy to the people who benefit most.
In addition, the early trial results of VEGFR plus PD-1/PD-L1 inhibitors in advanced GC/GEJC showed that the use of this treatment in earlier or later studies is associated with more favorable tumor response and survival outcomes. . Theoretically, this may be related to the tumor microenvironment with more immune tolerance in the early stage, and it is encouraged to verify this result in a larger pivotal phase III trial. Such a test can test this combination strategy alone in a sequential approach after chemotherapy or when used in combination with chemotherapy. If the survival advantage of this combination is confirmed in phase III clinical trials, the next step will be to combine this therapy with other multimodal therapies in early GC/GEJC.
Conclusion
By modulating the anti-tumor immune response, anti-angiogenic therapy for GC/GEJC patients appears to be a promising option to overcome resistance to IO therapy. The preclinical and clinical evidence reported to date indicates that the combined use of IO and VEGF targeting agents can improve overall efficacy. The mature survival results from the ongoing experiments are highly anticipated.
Progress and principle of combined immunotherapy for gastroesophageal cancer
Progress and principle of combined immunotherapy for gastroesophageal cancer
Progress and principle of combined immunotherapy for gastroesophageal cancer
Progress and principle of combined immunotherapy for gastroesophageal cancer
Progress and principle of combined immunotherapy for gastroesophageal cancer
(source:internet, reference only)
Disclaimer of medicaltrend.org



