Cross-protection mechanism of live attenuated influenza vaccine
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Cross-protection mechanism of live attenuated influenza vaccine
Cross-protection mechanism of live attenuated influenza vaccine. Influenza is an acute respiratory infectious disease caused by influenza virus, which infects 1 billion people worldwide every year. The burden of influenza disease is mainly concentrated in children and the elderly. Influenza outbreaks can also cause huge economic losses to society.
It is globally recognized that the most effective way to prevent influenza is to vaccinate against influenza. There are many factors that reduce the protective efficacy of influenza vaccines. The mismatch between vaccine strains and seasonal influenza virus epidemic strains is one of the main reasons. Fortunately, live attenuated influenza vaccines can be provided to recipients through cross-immunization. Protection to reduce this risk.
This article briefly introduces influenza virus inactivated vaccines and influenza live attenuated vaccines, as well as the cross-protection mechanism of influenza live attenuated vaccines.
1. Introduction to influenza virus vaccine
1.1 Inactivated influenza virus vaccine
The vaccine prepared by culturing the virus seeds for influenza vaccine production and inactivating by specific means is called influenza virus inactivated vaccine. At present, the influenza virus culture medium mainly includes chicken embryo and mammalian-derived cells around the world. The commonly used virus inactivating agent is formaldehyde or β-propiolactone. Inactivated influenza virus vaccines mainly include subunit vaccines, split vaccines and whole virus vaccines.
The strains used for the production of such vaccines are mainly provided by the World Health Organization (hereinafter referred to as WHO). The WHO estimates and announces the virus strains for the next influenza season for influenza vaccine production based on the monitoring of the global influenza epidemic every year.
However, the influenza vaccine prepared by this method of predicting the epidemic virus strain is uncertain. Once the vaccine strain does not match the epidemic strain, the vaccine will not be able to provide effective protection. So far, there have been many mismatch events.
The fundamental reason for the decline in the protection rate is that the main effective components of the influenza virus inactivated vaccine are hemagglutinin (hereinafter referred to as HA) and neuraminidase (hereinafter referred to as NA) proteins. These two proteins induce the body to produce neutralizing antibodies that are vaccines. The most important factor for the protective effect, and the HA protein is easy to mutate, resulting in new influenza virus subtypes, but the HA antigen stimulates the body to produce antibodies with strong specificity, and the neutralizing antibodies induced by this cannot effectively neutralize the mutants. Influenza virus cannot cope with the influenza pandemic caused by the mutation of the epidemic strain.
In addition to HA and NA proteins, influenza virus also contains a variety of proteins. The protein composition of influenza virus is shown in Figure 1.
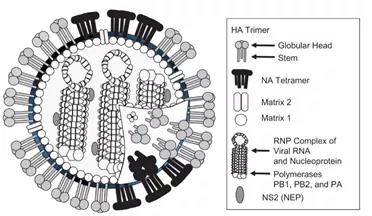
1.2 Live attenuated influenza vaccine
The virus species used for the production of live attenuated influenza vaccines is obtained by reproducing the current influenza epidemic strain recommended by WHO with the main donor strain with attenuating characteristics to obtain both HA and NA proteins of the epidemic strain and attenuating characteristics. Attenuated strain of influenza virus. The virus seed was inoculated into SPF chicken embryo allantoic fluid, cultured and proliferated, and purified to make influenza attenuated live vaccine.
This type of vaccine is vaccinated by nasal spray, making it the best choice for children who have difficulty receiving injections. After vaccination, the virus will proliferate in the nasal mucosa to a limited extent. This is a process that mimics natural infection and induces multiple immune responses including mucosal immunity to produce a combined protective effect. This is the main way in which live attenuated influenza vaccines work.
In addition, an interesting phenomenon can be observed: the occurrence of cross-immunity protection. There are many literature reports on this phenomenon: after volunteers were vaccinated with H7N3 live attenuated vaccine, when the H7N9 virus was prevalent, 44.8% of H7N3 live attenuated vaccine recipients detected positive conversion of antibodies against H7N9 virus[1, 2]; Young pigs can resist H1N2 infection after being vaccinated with H1N1 live attenuated vaccine [3]. More clinical trial results are shown in Table 1.
These test results show that the live attenuated influenza vaccine has cross-immune protection, and it is further speculated that the protection of the live attenuated influenza vaccine will be more extensive when the mutant influenza virus is pandemic.
Table 1. Cold-adapted influenza virus attenuated vaccine trials and clinical data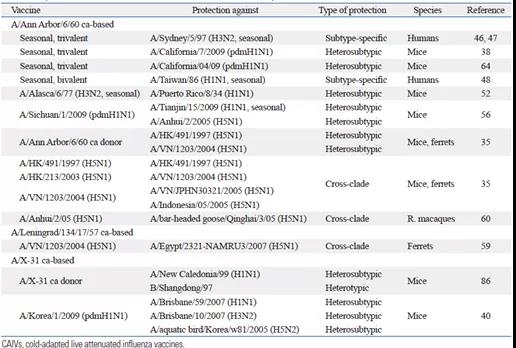
2. Reasons for cross protection
2.1 CTL reaction
The CTL response is a cytotoxic effect involving CD8+ T cells mediated by MHC-I molecules. When pathogens infect and invade the cells, this toxic CD8+ T cells can quickly kill the host cells and prevent the spread of the virus. Studies have shown that CTL response plays a key role in activating cross protection.
The activation of CTL depends on whether MHC-I molecules recognize effective antigens, and the antigens corresponding to MHC-I molecules in influenza viruses are mainly concentrated inside the virus, such as M1 protein and NP protein. These two proteins are only in the process of proliferation. Will be exposed, so inactivated influenza vaccine containing only HA and NA is difficult to activate the CTL response.
M1 is the matrix protein of influenza virus. According to literature reports, the renaturation test of peptides and HLA molecules has shown that after the binding of M1 specific T cell epitope peptides to HLA-A, it can promote the production of memory CD8+T, thereby enhancing CTL reaction. According to reports in the literature, the influenza A viruses circulating in 1918 and 2009 shared 6 identical CD8+ T cell epitopes on the M1 protein, indicating that the M1 protein is an extremely conserved protein, which is also its induction One of the reasons why the CTL has cross protection [4]. Figure 2 shows the formation of memory CD8+ T cells.
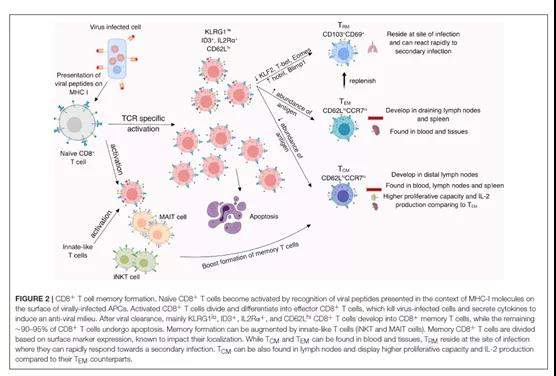
2.2 IgA
Through nasal administration, influenza virus will directly contact the mucosa of the upper respiratory tract. After the mucosa is infected, a large number of secretory IgA antibodies are produced [5]. Secretory IgA (sIgA) is the main effector of mucosal immunity, and sIgA also participates in stimulating the immune response. The reason why IgA exerts cross-protection is mainly based on the fact that secreted IgA is a kind of polymer. After a variety of specific IgA is polymerized, it can not only provide protection against a certain influenza virus, but also protect against the same influenza virus. Sources and even heterologous influenza viruses play a protective role [6]. These conclusions have been verified. For example, mice after nasal immunization with H1N1 (PR8) can resist PR8 homologous influenza infection [7].
2.3 The non-neutralizing antibody effect of NP protein
NP protein can also induce the body to produce specific IgG antibodies, but this IgG has no neutralizing activity. Studies have found that this IgG can promote the proliferation of memory CD8+ T cells, thereby enhancing the CTL response.
2.4 Neutralizing antibody effect
The specific antibody IgG against the HA protein is a molecule that plays a key role in the body’s fight against viral infections. There are multiple conserved sequences in HA, which also contain epitopes. The influenza vaccine attenuated strains contain HA and NA antigens of epidemic strains. Epitope, so its existence guarantees the most basic effectiveness of the vaccine. The possibility of further development of universal influenza vaccines through these sequences is currently being studied. Live attenuated vaccines are used to inject the whole influenza virus into the human body. The body’s immune system recognizes the virus more comprehensively than unit vaccines. It includes more conservative structural proteins. The antibodies induced by these structural proteins can also play a role in cross-neutralization protection. To a certain effect.
2.5 Innate immunity
Innate immunity can provide immediate protection when the body is infected by a live attenuated vaccine strain. Although this protection is non-specific and short-lived, it induces cytokines or chemokines to interfere with virus proliferation and activate non-innate immunity. Assist healthy cells to resist viral infections, etc. Studies have shown that all mice vaccinated with H3N2 live attenuated vaccine can resist H1N1 virus infection through the protection of innate immunity in the first 3 to 4 days [8].
3. Further research on live attenuated influenza vaccine
3.1 Replacement of the wild strain sequence
Because the NP protein is the key protein to activate the CTL response, some studies have replaced the NP protein in the attenuated strain with a wild strain, and found that it has no effect on the proliferation of the virus, and the cross-protection effect of the vaccine has been further strengthened [9]. There are also studies to modify the nucleic acid sequence of the HA protein to add more B cell receptor epitopes to enhance antibody neutralization or add CTL epitopes to enhance CTL response and further enhance cross-protection [10]. After the body is vaccinated with influenza vaccine, it will produce long-term memory B cells, but the antibodies secreted by these B cells are highly specific and cannot protect against mutant influenza viruses. Therefore, the vaccine can induce long-term memory T cells is the future It is a good choice to add ingredients that can effectively induce memory T cells in the vaccine.
3.2 Use of adjuvants
At present, there are many choices of adjuvants for influenza vaccine, such as MF59, QS21, AS02, Rintatolimod and so on. In terms of providing cross protection, Rintatolimod has good potential. It is a Toll-like receptor stimulant that will greatly increase the body’s secretory IgA antibody production. The body’s cross-protection ability is also significantly improved when different strains are used to challenge the virus[ 5]. At present, there are also applications of combined adjuvants in order to further increase the effectiveness of the vaccine.
Sum up
Live attenuated influenza vaccine can effectively activate innate immunity, CTL response and secretion of IgA antibodies. These factors work together to make the body’s immune protection not only specific, but also possible to have cross-protective effects. Further research such as adjuvants, genetic engineering, protein modification and other methods can further enhance the effectiveness of live attenuated influenza vaccines.
latest news:
On February 26, 2021, WHO officially released the influenza strains required for the 2021-2022 Northern Hemisphere influenza season vaccine, so that countries and vaccine manufacturers can develop and produce influenza vaccines. The WHO received a report on the 18th that Russia had found 7 cases of H5N8 avian influenza in human clinical samples.
In 2020, the H5N8 avian influenza virus was also found in poultry and wild birds in Bulgaria, Czech Republic, Egypt, Germany, Hungary, Iraq, Japan, Kazakhstan, the Netherlands, Poland, Romania, the United Kingdom and Russia.
The WHO stated that there may be new pandemics in the future, and it is paying close attention to potential pandemic threats to ensure that countries have good surveillance systems to quickly detect diseases and take countermeasures.
The variability of influenza viruses is still the biggest threat to the effectiveness of influenza vaccines. Before the universal influenza vaccine was born, the cross-protection mechanism of live attenuated influenza vaccines was the best solution to this problem.
It is recommended that the next technological upgrade of the live attenuated influenza vaccine can in-depth research on improving its cross-protection mechanism, expand the scope of cross-protection and improve the effect of cross-immunity.
(source:internet, reference only)
Disclaimer of medicaltrend.org



