Multiple Cardiac Progenitor Cell Populations in Mouse Embryonic Heart Region
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Multiple Cardiac Progenitor Cell Populations in Mouse Embryonic Heart Region
Multiple Cardiac Progenitor Cell Populations in Mouse Embryonic Heart Region. Science: New study identifies multiple cardiac progenitor cell populations in mouse embryonic heart regions.
The heart of vertebrates is composed of different types of cells, which are essential for normal heart function. In mice, the earliest mesoderm progenitor cells of cardiomyocytes are formed during gastrulation, migrate from the primitive streak to the rostral side, form a heart crescent, and begin to contract. The heart crescent shape is then reshaped to form a linear heart tube.
There are at least two different sets of mesodermal cardiac progenitor cells: the first heart field (FHF) and the second heart field (SHF). These two groups of mesoderm cardiac progenitor cells differ according to the early embryo However, the marker genes expressed in the overlapping regions are roughly determined.
Cells from outside these heart-producing areas also help the heart. One such structure, the proepicardium, produces the epicardium, which is the outermost layer of cells in the vertebrate heart. The epicardium provides important paracrine signals and can also produce a variety of heart cell types, including cardiomyocytes, vascular smooth muscle cells, and fibroblasts.
Scientists currently have limited understanding of when and how different heart cell types are produced during early development. Single-cell transcriptomics provides a powerful method to characterize the various cell types of the embryonic heart and generate hypotheses about their origin and fate.
Therefore, in a new study, researchers from the University of Oxford, Cambridge University, and the Helmholtz Munich Center in Germany combined single-cell RNA sequencing with high-resolution volume imaging and time-lapse microscopy to achieve single-cell resolution. To accurately characterize the cells of the mouse embryonic heart.
This powerful combination method provides a unified transcriptional and anatomical definition of cardiac progenitor cell types and their differentiation trajectories into cardiomyocytes. The relevant research results were published in the Science Journal on March 5, 2021, with the title of the paper “Characterization of a common progenitor pool of the epicardium and myocardium”.
Picture from Science, 2021, doi:10.1126/science.abb2986.
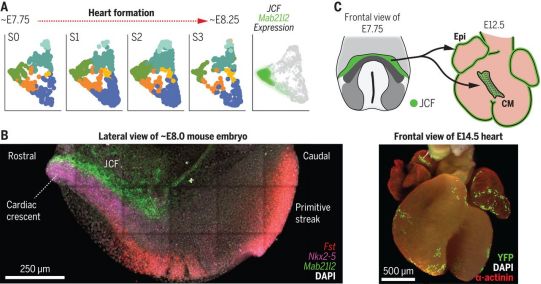
These authors used manual microdissection technology to isolate the heart region of mouse embryos, from the early heart crescent to the linear heart tube stage, and performed single-cell RNA sequencing. This allowed them to transcriptionally determine the cardiac progenitor populations in this region, including FHF and SHF. As a user-friendly community resource, they created a web interface to explore these data: https://marionilab.cruk.cam.ac.uk/heartAtlas/.
To determine the anatomical location of these cell populations at single-cell resolution, the authors used whole-mount immunohistochemistry, multiple fluorescence in situ hybridization, and high-resolution volume imaging of multiple markers. This comprehensive imaging analysis reveals the discrete locations of these transcription clusters and emphasizes the spatially ordered maturation of cardiomyocytes. It also identified a group of progenitor cells located in the crescent-shaped beak of the heart, located at the intersection of the embryo and the ecto-embryonic mesoderm, which they called the juxta-cardiac field (JCF). Using single-cell resolution time-lapse imaging and genetic lineage markers, they determined that JCF can contribute to the production of cardiomyocytes and epicardium. Therefore, JCF represents the earliest known epicardial progenitor cells.
It can be seen that this study described in detail the transcriptional state and anatomical location of cardiac progenitor cells, as well as their transitional state in the process of differentiation into cardiomyocytes, thus becoming a valuable community resource. In addition, it provides new insights into the formation of the heart. By identifying the pericardial area, this study broadened the area of cardiac progenitor cells and identified the earliest progenitor cells in the preepicardium. This research will help to better understand the origin of congenital heart defects and provide basic insights for the development of regenerative methods for the treatment of heart disease.
(source:internet, reference only)
Disclaimer of medicaltrend.org



