Stereotactic radiosurgery for patients with Koos IV vestibular schwannoma
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Stereotactic radiosurgery as the initial treatment for patients with Koos IV vestibular schwannoma
Stereotactic radiosurgery as the initial treatment for patients with Koos IV vestibular schwannoma. Many long-term research conclusions support the role of stereotactic radiosurgery (SRS) in the treatment of small-volume vestibular schwannomas (VSs), but its role in the treatment of large-volume tumors is still controversial.
Methods:
Between 1987 and 2017, the author performed a single SRS on 170 previously untreated Koos IV VSs patients (volume from 5-20 cm3). The median tumor volume was 7.4cm3. The median maximum diameter of the tumor was 27.5mm. All tumors compress the midfoot of the cerebellum and press the fourth ventricle to shift. There were 93 males and 77 females, with a median age of 61 years. 62 patients had normal hearing (Gardner-Robertson [GR] grade I and II), and the median peripheral dose was 12.5 Gy.
Results:
With a median follow-up of 5.1 years, the 3-year progression-free survival rate of VSs with peripheral doses ≥12.0 Gy was 98.4%, 95.3% at 5 years, and 90.7% at 10 years. In contrast, the tumor control rate after treatment with peripheral doses of <12.0 Gy was 76.9% at 3, 5, and 10 years. During SRS treatment, the 3-year hearing preservation rate of patients with normal hearing was 58.1%, the 5-year hearing preservation rate was 50.3%, and the 7-year hearing preservation rate was 35.9%. Younger patients (<60 years, p=0.036) and initial GR I (p=0.006) were associated with an increase in effective hearing preservation. Seven patients (4%) developed facial neuropathy during the follow-up period. Smaller tumor volume (<10cm3, p=0.002) and small peripheral dose (≤13.0 Gy, p<0.001) are related to the preservation of facial nerve function. When the peripheral dose is less than or equal to 13.0Gy, the probability of delayed facial neuropathy in 10 years is 1.1%. Nine cases (5%) required ventricular-abdominal shunt due to delayed symptomatic hydrocephalus. Trigeminal neuropathy occurred in 15 cases (9%). Delayed resection was performed in 4% of patients.
Conclusion:
Even for large-volume VSs, a single SRS can prevent nearly 90% of delayed resections within 10 years. For patients with low tumor space-occupying effect, SRS should be considered as an effective option outside surgery for most patients, especially those with advanced age or systemic diseases.
Vestibular schwannomas (VSs) are the most common benign tumors of the cerebellopontine angle. Stereotactic radiosurgery (SRS) for small and medium-sized VSs (usually Koos I or II) successfully prevented tumor growth in more than 95% of patients, and at the same time enhanced the protection of existing cranial nerve functions. SRS is also the first choice for young patients with large symptomatic VSs (Koos grade IV tumors) with compression of the cerebellar foot, brain stem and fourth ventricle, and no major complications after microsurgical resection. Although it is believed that potentially reactive peritumoral edema may occur, patients with large VSs and asymptomatic mass-occupying effects may be candidates for initial SRS treatment. Some research reports show that tumor control rates range from 79% to 94%. In these reports, some patients’ tumors have undergone surgery in the past but are still progressing.
The current retrospective results study aims to further improve the appropriate tumor peripheral dose, prolonged tumor response, cranial nerve prognosis, and the need for delayed surgical intervention in patients receiving initial SRS due to larger Koos IV VSs.
Methods:
In a single-center study at the University of Pittsburgh from 1987 to 2017, 1793 VSs patients received gamma knife treatment. Mezey et al. from a 30-year prospective study identified 170 patients with Koos IV VSs whose tumor volume was between 5.0-20.0 cm3 and all tumors compressed the midfoot of the cerebellum and displaced the fourth ventricle. Among them, patients with neurofibromatosis type 2 and patients who had received surgery or radiotherapy were excluded.
The upper limit of the selected volume is 20.0cm3, because according to experience, patients with tumors exceeding this volume have symptomatic mass effects, and tumor resection is usually the preferred treatment option. During this period, only 1 patient with a tumor volume> 20 cm3 received SRS as the initial treatment, and this patient underwent volumetric fractionated SRS treatment. The median volume of the tumor was 7.4 cm3 (range 5.0-20.0 cm3). The median diameter of the largest neural tube was 27.5 mm (21.1 -39.0 mm).
Among them, 93 were males and 77 were females. The median age of the patients was 61 years (19-88 years). Forty-seven patients (27.6%) had trigeminal sensation loss before SRS. Facial nerve function is classified according to House-Brackmann (HB) classification. Fourteen patients (8.2%) had facial neuropathy before SRS: HB grade was grade II (n=8) and grade III-VI (n=6). None of these patients had tumors extending to the facial nerve canal. Ninety-six patients (56.5%) had tinnitus before SRS, and 54 patients (31.8%) had imbalance or dizziness before SRS. 22 cases (12.9%) had mild gait disturbance.
All patients who reported hearing before SRS underwent hearing tests, including speech discrimination score (SDS) and pure tone average (PTA). Hearing status is classified according to the Gardner-Robertson listening power scale. There were 37 cases of GR grade I, 25 cases of grade II, 32 cases of grade III, 6 cases of grade IV, and 70 cases of grade V. Normal hearing was defined as GR I or II grade; 62 patients (36.5%) had normal hearing before SRS. Hearing deterioration is defined as a decline from GR I or II to III-V (SDS <50% and/or PTA> 50 dB).
The median follow-up time after SRS was 5.1 years (0.4-24.7 years); however, 9 patients were followed up for less than 1 year. Detailed patient characteristics are shown in Table 1.
Radiosurgery technology
The SRS process requires the installation of a stereotactic head frame under local anesthesia, and the patient undergoes enhanced MRI scans and localized CT scans. In the past 30 years, Leksell gamma knife (model U, B, C, 4C, Perfexion and Icon, Elekta AB) has been used for SRS. Use different versions of Leksell dose planning software (KULA or Leksell GammaPlan) for dose planning. The median peripheral dose was 12.5 Gy (range 10.5-22.0 Gy). The median maximum dose was 25.0 Gy (21.0 -45.0 Gy). Patients who received SRS before 1992 received higher doses (18.0-22.0 Gy).
The patient follow-up time is recommended for imaging examinations, hearing tests and clinical examinations every 0.5 year, 1 year, 2 years and every 4 years thereafter. When new symptoms or clinical changes appear, imaging examinations and follow-up should be carried out in a timely manner.
Data analysis
Use IBM SPSS statistics (version 25.0, IBM Corp.). The Kaplan-Meier chart of tumor control rate is constructed based on the SRS date and the date of tumor progression or the last imaging examination (if the tumor is under control). Tumor progression is defined as an increase in tumor volume by at least 15% over the volume in the initial dose plan.
To assess the volume of the tumor, multiply the measurement value of the control group by the maximum size of the 0.5 cm3 neural tube x, y, and z tumor. The Kaplan-Meier chart of the hearing preservation rate is based on the date of SRS and the date of hearing deterioration, or at the time of the last hearing examination, if the patient has no hearing deterioration.
Hearing deterioration is defined as a decline from GR I or II to III-V (not available, SDS <50% and/or PTA> 50 dB). The Kaplan-Meier chart of the probability of delayed facial neuropathy was constructed based on the date of SRS and the date of the last follow-up or the date of deterioration of facial function reported by the patient (HB grade decreased).
Kaplan-Meier log-rank statistical method was used for univariate analysis. Cox proportional hazards model was used for multi-factor analysis. The effects of the following clinical variables were studied: the patient’s age, gender, trigeminal nerve sensory loss at SRS, any level of facial weakness, hearing status, tinnitus, vertigo, gait disturbance, tumor volume and peripheral dose.
The recommended cut-off values of the variables (age, tumor volume, and peripheral dose) are determined by the Youden index of receiver operating characteristic curve analysis. The p value<0.05 is considered statistically significant.
Results:
Tumor control:
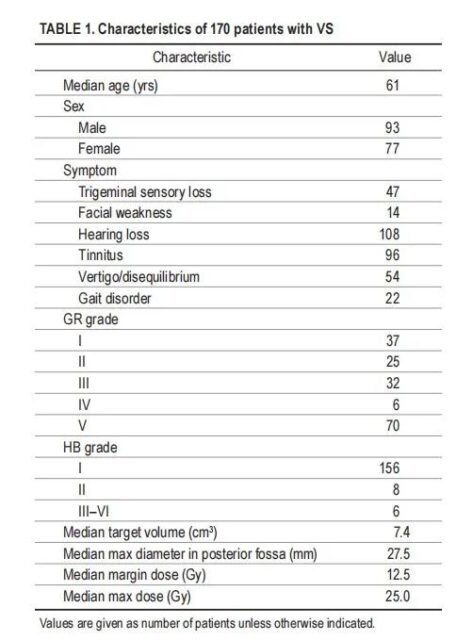
In this study, the prognosis of 170 Koos IV VSs patients who received SRS was studied. The total tumor control rate was 96.5% at 3 years, 93.7% at 5 years, and 89.4% at 10 years (Figure 1A). Eleven patients (6.5%) had imaging evidence of tumor enlargement. Three patients were under observation because their tumors remained stable after the initial treatment volume increased and there were no new symptoms or signs. One patient received additional SRS, after which the tumor remained stable. Seven patients underwent delayed tumor resection, including one patient with intratumoral hemorrhage.
The median interval between SRS and additional intervention was 38.1 months (8.4-88.9 months). In univariate analysis, we found that the following factors were not related to tumor control: age, gender, trigeminal nerve sensory loss, facial weakness, hearing status, tinnitus, dizziness, gait disorder, and tumor volume. Only a higher peripheral dose was significantly associated with increased tumor control (≥12.0 Gy, p = 0.004).
Similarly, in a multivariate analysis, only a higher peripheral dose was significantly associated with better tumor control (≥12.0 Gy, p = 0.008, HR 6.39, 95% CI 1.63-25.12; Table 2). The tumor control rate of VSs with peripheral dose ≥12.0 Gy for 3 years was 98.4%, 5 years was 95.3%, and 10 years was 90.7%.
In contrast, the tumor control rate for peripheral doses <12.0 Gy was 76.9% at 3, 5, and 10 years (Figure 1B). For patients with larger VSs or patients with hearing symptoms, the peripheral dose is less than 12.0Gy, and the peripheral dose can be reduced to minimize the cochlear dose.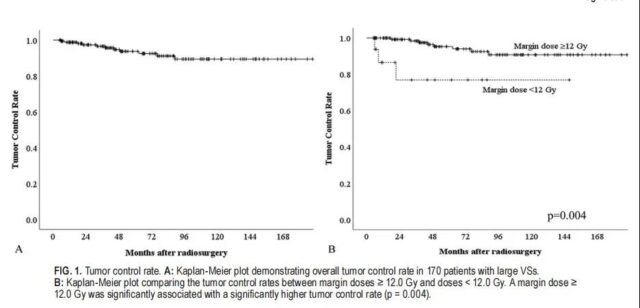
The degree of freedom for additional treatments is also evaluated in the study. In the multivariate analysis, only the peripheral dose was significantly associated with better tumor control (≥12.0 Gy, p=0.003, HR 9.50, 95% CI 2.20-41.00).
Useful hearing protection
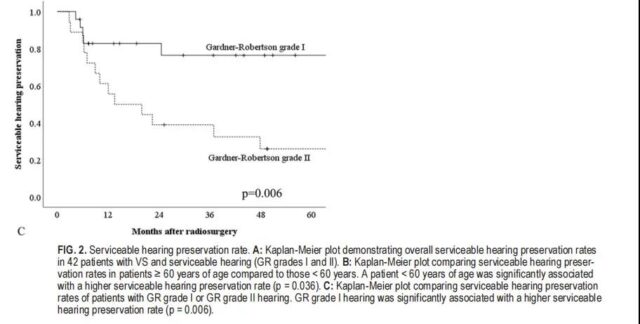
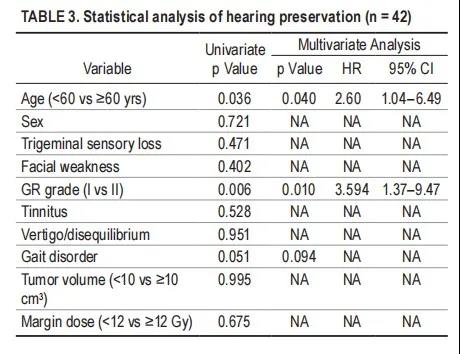
In this study, 42 of 62 patients with normal hearing before SRS (GR grade I and II) were reassessed during follow-up. The median long-term hearing follow-up time was 75.8 months. The overall hearing preservation rate was 58.1% at 3 years, 50.3% at 5 years, and 35.9% at 7 years (Figure 2A). In univariate analysis, we found that the following factors are not related to hearing preservation: gender, trigeminal nerve loss, facial weakness, tinnitus, vertigo, gait disorder, tumor volume and peripheral dose. Younger patients (< 60 years, p = 0.036) and better hearing when SRS (GR grade I, p = 0.006) and improved hearing protection rate (Figure 2b and C, Table 3). Multivariate analysis confirmed that younger patients (< 60 years old, p = 0.040, HR 2.60, 95% CI 1.04-6.49) and GR I (p = 0.010, HR 3.594, 95% CI 1.37-9.47) and effective hearing preservation Improve the correlation (Table 3).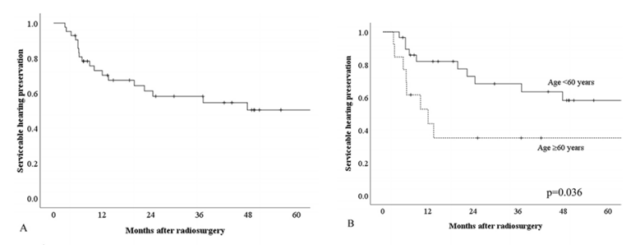
Results of facial nerves
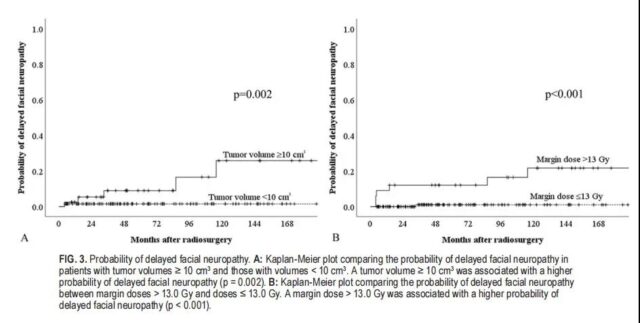
In this study, 7 patients (4.1%) developed delayed facial neuropathy after SRS. The probability of transient facial numbness after 10 years is 7.6%. In univariate analysis, we found that the following factors have nothing to do with the possibility of delayed facial neuropathy: age, gender, trigeminal nerve loss, facial neurasthenia, hearing status, tinnitus, vertigo, gait disorder. Smaller tumor size (<10cm3, p = 0.002) and lower peripheral dose (≤13.0 Gy, p<0.001) significantly reduce the risk of facial neuropathy (Table 4). Multivariate analysis showed that the tumor volume was smaller (<10<10cm3, p = 0.026, HR 6.51, 95% CI 1.26–33.73) and the peripheral dose was lower (≤13.0 Gy, p=0.011, 16.04HR, 95% CI1 .89-136.42) was significantly related to the preservation of facial nerve function (Table 4). Six of the seven patients with facial neuropathy received a peripheral dose of >13.0 Gy before 1992. One patient developed HB II facial neuropathy 33 months after receiving the 11.5 Gy dose. The probability of delayed facial neuropathy for 10 years with Koos IV and peripheral dose ≤13.0 Gy was 1.1%.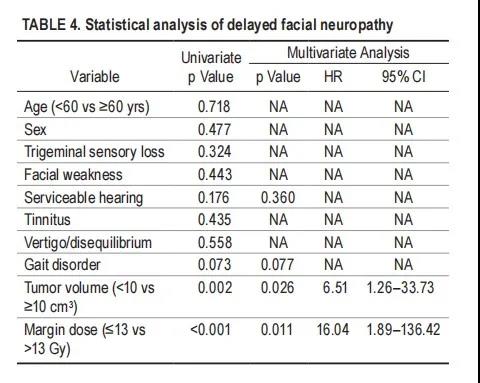
Results of the trigeminal nerve
Trigeminal neuropathy is defined as an increase in ipsilateral trigeminal sensory loss or the development of trigeminal neuralgia. Fifteen patients (8.8%) developed trigeminal neuropathy after SRS, of which 5 had worsening of the original symptoms, and 10 had new trigeminal symptoms. Nine patients developed trigeminal neuralgia and were initially treated with medication. One patient with intractable pain underwent both glycerol amputation and trigeminal nerve targeting SRS. As a result, larger tumor volume (< 10vs≥10cm3, p=0.798), higher peripheral dose (≤13.0vs>13.0 Gy, p=0.382), facial numbness before SRS (p=0.492) and trigeminal neuropathy The development has nothing to do.
hydrocephalus
After SRS, 8 patients developed hydrocephalus and required shunting, although the tumor volume did not change. One patient underwent tumor resection due to tumor progression, and required shunt surgery due to worsening hydrocephalus after surgery. The median time between SRS and shunt was 7.2 months (range 1.3-32.7 months). SRS tumor volume has nothing to do with the incidence of hydrocephalus (<10vs≥10cm3, p=0.520).
Tinnitus, dizziness, imbalance
Ninety-six patients (56.5%) reported tinnitus before SRS. During the follow-up, 15 patients (15.6%) had improved tinnitus or In remission, 76 cases (79.2%) remained unchanged, and 5 cases (5.2%) had worsening tinnitus. Among 74 patients without tinnitus before the onset, 4 cases (5.4%) developed tinnitus after the onset. 54 patients (31.8%) reported intermittent dizziness or imbalance before SRS. The symptoms of the vestibular fossa were improved or alleviated in 17 patients (31.5%), there was no change in 34 patients (63.0%), and the symptoms of the vestibular fossa worsened in 3 patients (5.6%). Among 116 patients, 7 patients (6.0%) had no vestibular fossa symptoms before SRS and had vestibular symptoms after SRS.
Gait disorder
22 cases (12.9%) had mild gait disturbance. Nine cases (40.9%) showed improvement, 11 cases (50%) showed no significant change, and 2 cases (50%) experienced deterioration of gait disturbance. 1 case underwent surgical resection due to tumor progression. One patient was recommended to undergo surgical resection due to worsening gait, but the patient refused the operation. In this experience, no patients were found to have malignant tumor transformation or development into radiation-related tumors during the follow-up period.
Discusion:
VSs are usually slow-growing benign tumors that originate from the vestibular nerve. The purpose of SRS is to prevent tumor growth, protect cranial nerve function, and avoid the risk of major intracranial surgery. At the time of diagnosis, the initial treatment options include further observation, surgical resection, SRS or fractional radiotherapy, and more recently stereotactic therapy, called stereotactic radiotherapy (SRT). In the past 30 years, SRS has been proven to be an effective and risk-reducing strategy for patients with small and medium-sized VSs. More and more documents confirm that compared with initial surgical resection, initial SRS has a better hearing retention rate and reduces the risk of facial neuropathy.
Patients with larger tumors may have more severe symptoms, including headaches, imbalances, gait disturbances, and progressive cranial nerve dysfunction. Patients who meet the initial complete or partial tumor resection conditions can relieve the early symptoms of cerebellar peduncle and brain stem compression. We found that although imaging showed large tumors, there was very little clinical evidence of symptomatic mass effects in some patients. Other patients may have mild symptoms, but due to their age or comorbidities, there is a significant risk of perioperative mortality or morbidity. For such patients, even for specific patients with large VSs, the results indicate that SRS may be an effective and safe strategy.
Tumor growth control
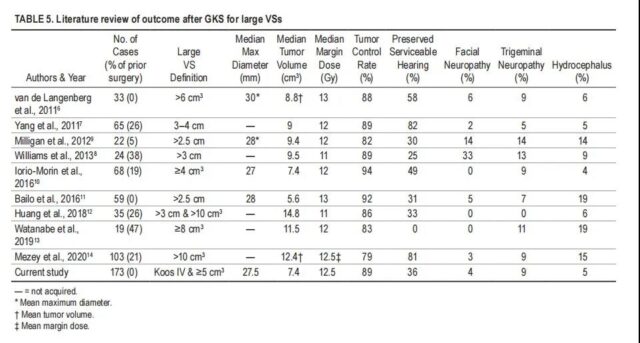
Previous studies have reported that the tumor control rate of SRS in the treatment of large VSsd ranges from 79% to 94% (Table 5). The study of the rate of difference in tumor control seems to be due to the different definitions of large VSs and the inclusion of patients who had previously undergone surgery or type 2 neurofibromatosis. We found that only two previously published studies evaluated SRS as the initial treatment for large VSs. Bailo et al. performed SRS on 59 VSs patients with tumor diameters ≥ 25 mm, using a median peripheral dose of 13.0 Gy, and reported a 92% tumor control rate. Van de Langenberg et al. treated 33 VS patients with a tumor volume> 6 cm3 with an average peripheral dose of 12.6 Gy, and reported a tumor control rate of 88%. In this study, we found that the 10-year tumor control rate was 89.5%. Only the peripheral dose ≥ 12.0 Gy is significantly related to the tumor control rate. The control rate of patients with tumor resection margin ≥12.0 Gy was 98.4% at 3 years, 95.3% at 5 years, and 90.7% at 10 years.
Hearing preservation rate
Previous studies have shown that for patients with larger VSs, the effective hearing preservation rate after SRS is quite different (0%-82%; see Table 5). Under normal circumstances, the hearing preservation rate decreases with the extension of follow-up time. . Several factors are thought to affect the preservation of hearing, including younger age, better initial hearing, smaller tumor size, lower peripheral dose, and lower dose delivered to the cochlea. In this study, younger patients (<60 years old) and GR I were associated with an increase in the effective hearing preservation rate. Young patients with better hearing can better maintain useful hearing after SRS.
Complication
In previous studies of SRS in the treatment of large VSs, the risk of facial neuropathy ranged from 0% to 33% (Table 5). In a recently published series of studies, using 12.0-13.0 Gy of the tumor peripheral dose showed that the risk of new facial neuropathy ranges from 0% to 2.7%. In this study, the probability of delayed facial neuropathy 10 years after SRS was 7.6%. Various published articles have shown that smaller tumor volume, smaller peripheral dose, and no previous surgical history are related to reducing the risk of facial nerve dysfunction. In the current experience, we found that smaller tumor volume (<10cm3) and smaller peripheral dose (≤13.0Gy) are significantly related to reducing the occurrence of delayed facial neuropathy. There were 2 patients with facial neuropathy more than 5 years after surgery. These 2 patients had larger tumors (≥10cm3) and were treated with higher peripheral doses (>13.0Gy).
Previous literature found that the larger VSs had trigeminal neuropathy in 0%-14%, and hydrocephalus required shunting in 4%-19% (Table 5) Lee et al. reported that a larger tumor volume increased the brain The risk of stagnant water and lead to diversion in an average of 15.5 months. In the current study, the tumor volume at the SRS is not related to the risk of symptomatic hydrocephalus. In our study, the median interval between SRS and shunt was 7.2 months. For patients with larger VSs, it is particularly important to monitor the symptoms of hydrocephalus in the first year after SRS.
Only a few literatures have reported symptoms of gait disturbance in patients with larger VSs, and 11%-23% of patients have been found to have such symptoms. In our study, only 2 patients experienced gait deterioration. Among patients with reduced tumors, 41% of patients had improved gait disturbance.
Our previous report stated that SRS with a peripheral dose of 12.0-13.0 Gy has higher tumor growth control rate, higher hearing preservation rate, and can reduce the incidence of facial and trigeminal nerves. Previous reports have reservations about the use of large VSs with peripheral doses exceeding 11 Gy. According to the treatment results of patients with large VSs, peripheral doses ≥12.0 Gy can improve tumor control, but the risk of facial neuropathy is lower in peripheral doses ≤13.0 Gy. For large VSs (tumor volume 5.0-20.0 cm3), we recommend a peripheral dose of 12.0-12.5 Gy.
Shortcomings of current research
During the 30-year long-term research, imaging and dose planning technology has been steadily developed. We do not routinely measure the radiation dose to tissues outside the tumor volume. Although these measurements are currently possible, we cannot yet correlate these volumes with clinical results, imaging evidence of increased reactive signals with long repetition times, or the risk of hydrocephalus. In follow-up reports, we hope to extend the median follow-up time. We are currently evaluating whether the percentage of tumor volume receiving more than 14-15 Gy may be a more important predictor of long-term tumor control. This research is based on the evolution of Leksell’s gamma knife usage over the past 30 years. It cannot answer whether other stereotactic radiation irradiation methods can achieve similar or improved results. Standard segmentation SRT (FSRT) and large segmentation SRT have been suggested as alternatives to SRS. There is no significant forward-looking data to confirm the hypothesis that segmentation improves results or reduces risk. Casentini et al. reported that 33 patients with large VSs (volume 8-24 cm3) received Cyberknife treatment (2-5 divisions, 14-19.5 Gy); in this study, the tumor control rate after 5 years of FSRT was 83%.
Conclusion:
For patients with large VSs (tumor volume 5.0-20.0 cm3), a single SRS with a peripheral dose of 12.0-13.0 Gy can obtain a higher long-term tumor control rate and satisfactory cranial nerve preservation rate.
For elderly patients or patients with major clinical complications, SRS should be considered as an alternative to initial surgical resection.
(source:internet, reference only)
Disclaimer of medicaltrend.org



