Stereotactic radiosurgery to treat cerebral arteriovenous malformations.
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Stereotactic radiosurgery combined with embolization to treat cerebral arteriovenous malformations.
Stereotactic radiosurgery combined with embolization to treat cerebral arteriovenous malformations. In January 2021, “Neurosurgery” published the work “Whether Stereotactic Radiosurgery Combined with Embolization to Treat Cerebral Arteriovenous Malformations” (DOI: 10.1093/neuros/nyaa418) written by Professor Jason P. Sheehan of Neurosurgery of Virginia Medical Center.

Overview:
Large cerebral arteriovenous malformations (AVMs) (diameter> 3cm or volume> 12ml) alone use standard stereotactic radiosurgery (SRS), the occlusion rate is lower than that of small AVMs. Neoadjuvant embolization has been used to shrink the focus of large AVMs before receiving SRS treatment. In addition, embolization has also been used for high-risk structures associated with AVM, such as aneurysms in and around malformations or high-flow arteriovenous fistulas. More and more evidence alludes to the negative impact of AVM embolization after SRS treatment on the occlusion rate.
However, the main limitation of these studies on the relationship between embolization and SRS treatment of AVM occlusion rate is that the original size of the untreated AVM cannot be obtained (for example, the volume reduced by embolization or the change of vascular architecture). The purpose of this multicenter, retrospective, paired study is to compare the results of SRS with or without neoadjuvant embolization for AVMs based on the characteristics of new-born AVMs.
Methods:
This study followed the STROBE statement, and through the ethics of each center, informed consent was exempted. Institutions within the International Radiosurgical Research Foundation (IRRF) were invited to provide AVM cases treated with a single course of SRS between 1987 and 2018 for this study. Patients were divided into two cohorts according to whether they were embolized before SRS: (1) embolization combined with SRS group (E+SRS cohort) (2) SRS treatment group alone (SRS cohort). Then the two groups were matched 1:1 by propensity score. The primary endpoint event is the occlusion of AVM. Secondary endpoints were postoperative bleeding, all-cause death, imaging and symptomatic radiological response (RIC), and cystic change after SRS.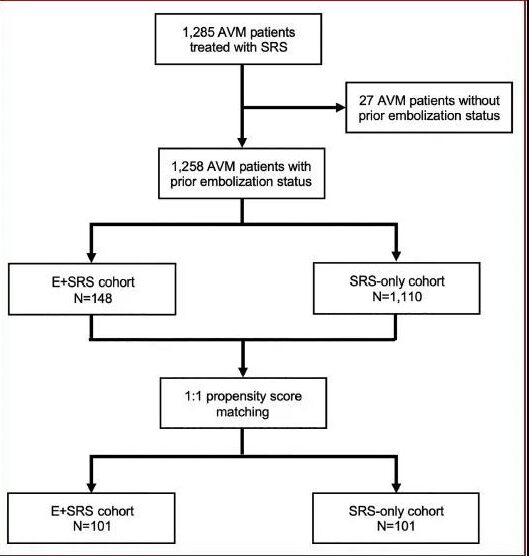
Results:
Baseline characteristics of unmatched cohorts
After excluding patients with unknown previous embolism status, the entire study cohort included 1258 AVM patients receiving SRS treatment. The E+SRS group and SRS group included 148 and 1110 patients, respectively.
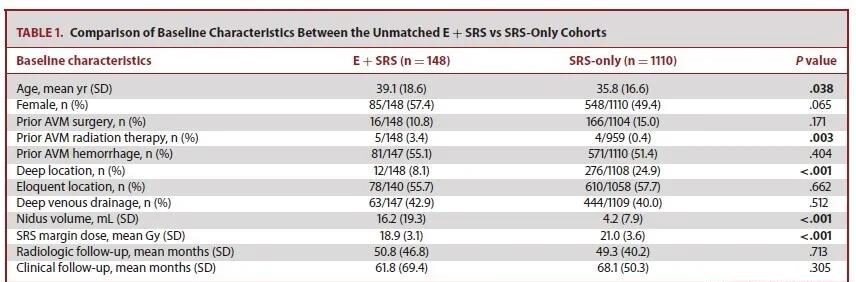
Table 1 compares the baseline characteristics of mismatched patients in the two cohorts. Compared with SRS alone, E+SRS patients have larger AVMs (16.2 vs 4.2ml), fewer patients with deep deformities (24.9% vs 8.1%), and lower marginal doses (21Gy vs 18.9Gy).
Prognosis of unmatched cohort
Table 2 compares the prognosis of the two unmatched cohorts.
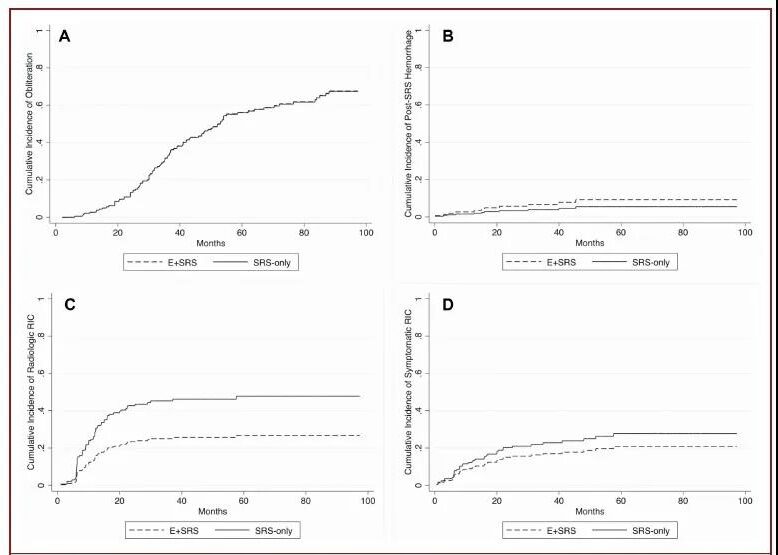
The occlusion rate of AVM: E+SRS group was lower than SRS alone (43.4% vs 64.0%, P <.001). The cumulative occlusion rate of the E+SRS group at 3, 4, 5, and 6 years was lower than that of the SRS group (31.1%vs43%, 43.8%vs58.1%, 49.8%vs64.7%, 54%vs69%).
The incidence of bleeding: the two groups of unmatched cohorts had similar rates of bleeding at 3, 4, 5, and 6 years after surgery, and there was no statistical difference (P=0.300). (E+SRS group: 5.9%, 7.7%, 9.5%, 7.2%; SRS group: 4.2%, 5.5%, 6.8%, 7.2%). The rough rates of all-cause mortality were similar between unmatched cohorts (P=0.990)
Radiation-induced changes (RIC, radiation-induced changes): The two groups had similar probability of RIC that was visible on the image at 3, 4, 5, and 6 years after surgery, and there was no statistical difference (P=0.154). (E+SRS group: 29.0%, 29.7%, 31.1%, 31.4%; SRS group: 35.3%, 36.2%, 37.8%, 38.2%). The cumulative symptomatic RIC for 3, 4, 5, and 6 years of the E+SRS group was higher than that of the SRS group (20.9%vs13.6%, 22.5%vs14.6%, 23.6%vs15.5%, 24.2%vs15.5% , P=0.021).
Capsule degeneration: The probability of cyst degeneration in the E+SRS group was higher (3.6% vs 1.2%; P=0.035). The cumulative probability of cystic transformation at 3, 4, 5, and 6 years after surgery in the two unmatched cohorts was similar (E+SRS vs SRS: 0.7% vs 0.3%, 0.7% vs 0.3%, 1.9% vs 0.8%, 3.3%vs1.3%; P=0.061).
Baseline situation of the matched cohort

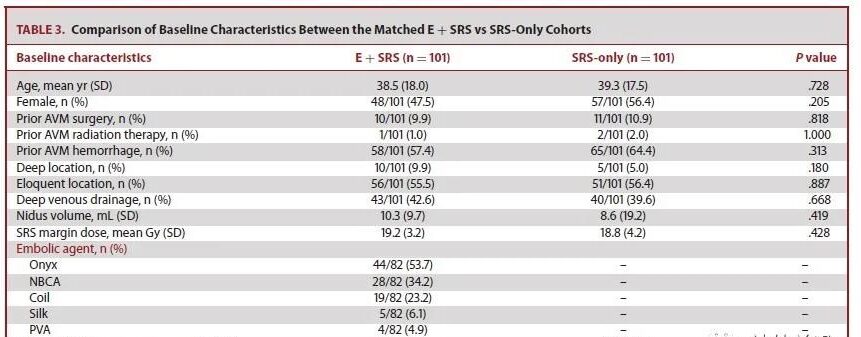
101 patients were matched in each cohort. The baseline indicators are stable. Table 3 shows that the baseline conditions of the two cohorts after matching are stable. The most commonly used embolic materials in the E+SRS group: Vinyl alcohol copolymer (Onyx®) accounted for 53.7%, cyanoacrylate (NBCA) accounted for 34.2%, and coils accounted for 23.3%. Among the data available for analysis in the matched E+SRS cohort, pre-SRS embolization only occluded aneurysms in 3.7% (n=2/57).
Prognosis of matched cohort
Table 4 and Figure 3 compare the prognosis of the two cohorts after matching.
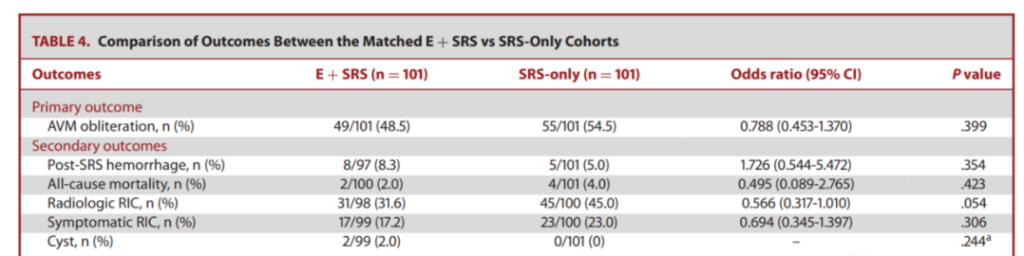
The occlusion rate of AVM: After matching, the occlusion rate of the two cohorts was similar, and there was no statistical difference (P=0.399). The proportions of E+SRS and SRS cohorts who completed DSA were 70.3% (n=71/101) and 60.4%, respectively (N=61/101). The cumulative occlusion rates of 3, 4, 5, and 6 years are similar, with no statistical difference (E+SRS vs SRS: 33%vs32.9%, 46.4%vs46.2%, 56.2%vs56%, 60.8%vs60.6 %; P=0.981).
The incidence of bleeding: the probability of postoperative bleeding after matching the two cohorts was similar, and there was no statistical difference (P=0.354). The cumulative probability of bleeding at 3, 4, 5, and 6 years after surgery was also similar, and there was no statistical difference (P=0.334). (E+SRS group: 6.6%, 9.2%, 9.2%, 9.2%; SRS group: 3.9%, 5.5%, 5.5%, 5.5%). The rates of all-cause mortality between unmatched cohorts were similar (P=0.423).
Radiation-induced changes (RIC, radiation-induced changes): The probability of RIC (P=0.054) and symptomatic RIC (P=0.306) visible on the images of the matched two cohorts is similar. However, in the E+SRS cohort 3, 4, 5, and 6 years after surgery, the cumulative probability of visible RIC was lower (25.0%, 25.7%, 26.7%, 26.7% in the E+SRS group; 45.3% in the SRS group , 46.2%, 47.8%, 47.8%). However, the probability of symptomatic RIC was higher in the E+SRS group (20.3% vs 13.2%, P=0.024). The cumulative probability of symptomatic RIC in the two cohorts was similar (E+SRS vs SRS: 17.0%vs22.8%, 18.7%vs25.0%, 20.9%vs27.8%, 20.9%vs27.8%, P=0.308 ).
Cyst degeneration: After matching, the probability of cyst degeneration in the two cohorts was similar (P=0.244). Embolism-related asymptomatic and symptomatic complications in the E+SRS group were 8.3% (n=8/97) and 18.6% (n=18/97), respectively. Among the patient data of embolism-related complications available for analysis, one case developed asymptomatic imaging visible RIC, and the other developed symptomatic RIC.
Discussion:
For large AVMs, embolization is a recognized auxiliary method to reduce the size, and it has also become an intervention for the purpose of promoting SRS to treat small AVMs. A large number of previous studies have shown that embolization combined with SRS reduces the occlusion rate of AVM. A meta-analysis of 12 studies showed that the occlusion rate of 681 AVMs who received embolization combined with SRS was lower than that of 978 who received SRS alone (48% vs 63%, P<0.00001). However, patients receiving E+SRS used the characteristics of AVM parameters after embolization. Therefore, these comparisons are biased because patients receiving embolization may have a larger initial volume of AVM and more complex vascular architecture than patients receiving SRS alone. In order to compare the SRS results of embolic and non-embolism AVM, the characteristics of the initial and pre-embolism AVM must be analyzed.
Main findings-conclusion
AVM embolization has no negative effects on post-SRS occlusion, post-SRS bleeding and overall complications. Embolization before SRS seems to provide some protection against adverse radiation effects, but this potential benefit may be offset by the risks of AVM neurovascular intervention. The results of this study refute the popular belief that early lesion embolization has a negative impact on SRS-induced occlusion of AVMs. In the multimodal management of AVM with large volume or high-risk vascular architecture, neoadjuvant embolization will not increase the occlusion rate of AVMs after SRS. Therefore, when using this treatment strategy, appropriate AVMs should be carefully selected.
(source:internet, reference only)
Disclaimer of medicaltrend.org



