Collection: FDA oncolytic virus product clinical research summary
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
Collection: FDA oncolytic virus product clinical research summary
Collection: FDA oncolytic virus product clinical research summary. Recently, “Biomedicines” recently released a summary of the progress of oncolytic virus clinical trials. As a new type of treatment of malignant tumors, oncolytic virus has achieved phased results in clinical trials of various cancer treatments. The researchers detailed the anti-tumor effects of the four major types of oncolytic viruses in clinical trials as a single treatment or combined treatment.

About oncolytic viruses
Oncolytic virus (OV) is a wild-type or engineered virus that can selectively replicate and kill cancer cells in cancer cells without harming normal cells. As a new type of multi-mechanism cancer treatment method, OVs can directly lyse cancer cells and activate anti-tumor immune responses to “chall poison with poison”. It has the advantages of good targeting, strong lethality, and not easy to tolerate.
Clinical studies have shown that oncolytic viruses can bring clinical benefits to cancer patients of different types and stages of progression. When combined with chemotherapy and immunotherapy drugs, the synergistic effect is significant.
Currently, there are four common viruses to which oncolytic drugs belong: herpes virus (HSV), vaccinia virus (VV), adenovirus (Adeno), and reovirus (REO).
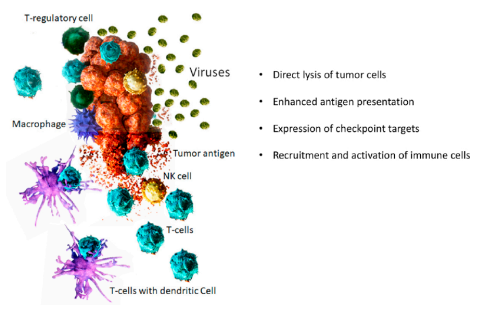
(The picture shows the anti-cancer mechanism of oncolytic virus)
01. Herpes virus (HSV)
Herpes virus (HSV): Encapsulated double-stranded DNA virus, in a spherical shape. The complete virus is composed of a core, capsid, tegument, and envelope. The core contains double-stranded DNA, which is wound into a filament reel. The capsid is icosahedral symmetrical and consists of 162 shell particles with a diameter of 100nm.
The outer layer of the capsid is covered by a membrane with uneven thickness. The outermost layer is a typical lipid bilayer membrane with protrusions. The diameter of the enveloped virus is 150 to 200 nm. It infects a wide range of hosts, mainly affecting the skin, mucous membranes and nerve tissues.
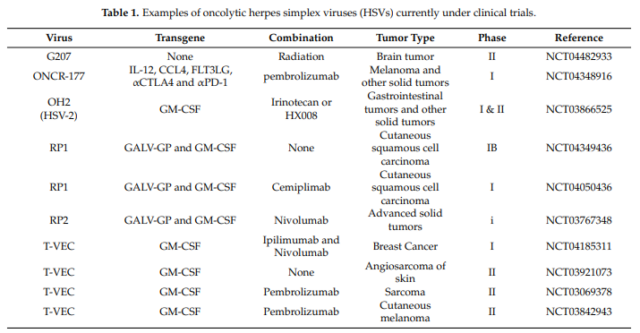
01) T-VEC
T-VEC (Imlygic) is a marketed product of Amgen, which was approved by the US FDA in 2015 for the local treatment of melanoma patients who recurred after surgery.
T-VEC was originally a JS-1 strain isolated from the cold sore virus. Due to the deletion of two copies of the neurotoxic gene ICP34.5, the virus is weakened. Enhanced virus-mediated immune destruction by deleting gene ICP47 (encoding major histocompatibility complex MHC I-mediated antigen presentation inhibitor); enhanced by inserting granulocyte-macrophage colony stimulating factor (GM-CSF) This improves the recruitment and activity of dendritic cells, and ultimately enhances anti-tumor immunity.
Clinical trial subjects: patients with refractory melanoma, breast cancer, gastric cancer or head and neck cancer
Monotherapy stage I: intratumoral injection of 10^6PFU virus, the treatment is well tolerated, and the side effects are local inflammation and febrile erythema. The results show that the virus can replicate in tumors and express exogenous GM-CSF.
Stage II: 50 patients with stage IIIC unresectable or metastatic melanoma received intratumoral injection of 10^8PFU virus. It can be observed that the injected tumors and non-injected metastatic tumors regression, the overall response rate (ORR) is 26%, and 8 of the 13 responding patients achieved a complete response. It is well tolerated and side effects are inflammation at the injection site, flu-like symptoms, and nausea.
Stage III: In 436 patients with stage III to IV melanoma, the ORR of intratumoral T-VEC treatment was 31.5%, and 16.9% had a complete response.
Combination therapy:
①T-VEC combined with ipilimumab (CTLA4 checkpoint inhibitor) to treat patients with advanced melanoma. The overall survival rate at 18 months was 67%, which was higher than T-VEC or ipilimumab monotherapy.
②T-VEC combined with pembrolizumab (anti-PD-1 antibody) in the treatment of patients with advanced melanoma, the complete remission rate was 33%.
02) G207
G207: Same as T-VEC, two copies of the ICP34.5 gene are deleted, and the ICP6 gene is inactivated by inserting the E. coli lacZ gene.
Clinical trial object: brain tumor
Monotherapy stage I: 21 patients with glioblastoma multiforme, intratumoral injection of 10^6~3E+10^9PFU virus clinically showed anti-tumor activity.
Combination therapy: 9 patients with relapsed malignant glioma received intratumoral injection of 10^9PFU+5Gy radiotherapy, 66.6% partial response.
03) G47△
G47△: A variant of G207 with an additional deletion in gene ICP47, which can enhance MHC-I presentation on infected cells.
Clinical trial object: patients with glioblastoma
Monotherapy Stage II: The 1-year survival rate (92.3%) of 13 Japanese glioblastoma patients was significantly higher than that of the control group (15%).
04) ONCR-177
ONCR-177: The oncolytic HSV-1 virus developed by Oncorus, which inserts tissue-specific microRNAs (miRNA) sequences into the early genes (ICP4, ICP27, UL8) and ICP34.5 genes of the virus genome, so that the virus can be It replicates only in tumor cells, but not in normal cells. Furthermore, the UL37 gene was mutated to prevent retrograde axon transport and incubation period to ensure that the virus does not damage neurons. Phase I single and combined trials are in progress.
05) RP1/2
RP1/2: deletion of genes ICP34.5 and ICP47, insertion of GM-CSF and gibbon leukemia virus glycoprotein (GALV-GPR-). RP2 expresses anti-CTL4.
Clinical trial subjects: patients with recurrent or advanced cutaneous squamous cell carcinoma
Phase I single and combined trials are in progress.
02 Vaccinia virus (VV)
Vaccinia virus, enveloped double-stranded DNA virus, is the largest type of DNA virus with virions. The virions are brick-shaped or oval in size (300-450) nm×(170-260) nm. There are core, side body and envelope. The core contains viral DNA bound to protein.
The clinical trial information is summarized as follows:
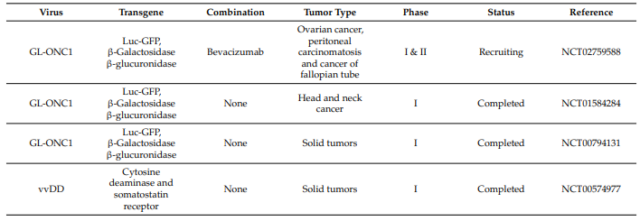
01) Pexa-Vec(JX-594)
Pexa-Vec (JX-594): Delete gene J2R, insert human GM-CSF (hGM-CSF) and E. coli lacZ, to improve tumor specificity and anti-tumor immune activity.
Clinical trial subjects: patients with refractory primary or metastatic hepatocellular carcinoma, patients with advanced refractory hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC)
Monotherapy Phase I:
①14 patients with refractory primary or metastatic hepatocellular carcinoma received intratumoral injection of 10^8PFU-3E+10^9PFU virus. The treatment showed that the patients who received the highest dose (3E+10^9PFU) showed hyperbilirubinemia, 3 cases were partially remitted, 6 cases were stable, and 1 case progressed, indicating that the MTD of JX-594 was 1E+10 ^9PFU.
②3 patients with advanced refractory hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) were injected intratumorally with the highest dose of 3E+10^9PFU virus. Anti-tumor efficacy was observed in all patients, inhibiting the replication of hepatitis B virus.
Stage II: 30 patients with advanced liver cancer, 10^8PFU (low dose) and 10^9PFU (high dose) were injected intratumorally on days 1, 15 and 29, respectively. The treatment showed the survival time of patients receiving high dose treatment (medium). The survival time of the patient was 14.1 months) was significantly higher than that of patients receiving low-dose treatment (6.7 months).
Combination therapy: JX-594 combined with kinase inhibitor Sorafenib in the treatment of HCC has better anti-tumor efficacy than single therapy. Phase III trials are in progress.
02) GL-ONC1(GLV-1h68)
GL-ONC1 (GLV-1h68): Developed by Genelux. GL-ONC1 deletes genes J2R, F14.5L and A56R, and inserts genes encoding β-galactosidase, β-glucosidase and fluorescent protein. Monotherapy in Phase I and II trials.
Clinical trial subjects: patients with colon cancer, breast cancer, glioma, ovarian cancer, pancreatic cancer and prostate cancer
04) vvDD (JX-929 or vvDD-CDSR)
vvDD (JX-929 or vvDD-CDSR): Western strain WR of VACV. Deleting the J2R and C11R genes allows the virus to selectively replicate in cells with active E2F and epidermal growth factor receptor (EGFR/Ras) pathways.
Clinical trial subjects: patients with colon cancer, ovarian cancer and liver metastases
Monotherapy Phase I:
① The maximum dose of 3E+10^9PFU, WR strain is well tolerated, and no clinical benefit has been achieved.
②11 patients with advanced colorectal or other solid tumors received intravenous injection of up to 3E+10^9PFU virus. It is well tolerated, and the adverse reaction is grade 1/2 flu-like symptoms. Tumor-specific replication of the virus has been observed, with minimal clinical benefit.
03. Adenovirus
Adenovirus (Adenovirus): non-enveloped double-stranded DNA virus, no envelope structure, 70-90 nm in diameter, composed of 252 capsid particles arranged in a icosahedron shape. The diameter of each shell particle is 7-9 nm. Inside the capsid is a linear double-stranded DNA molecule, about 4.7 kb, with inverted repeats of about 100 bp at each end.
The clinical trial information is summarized as follows: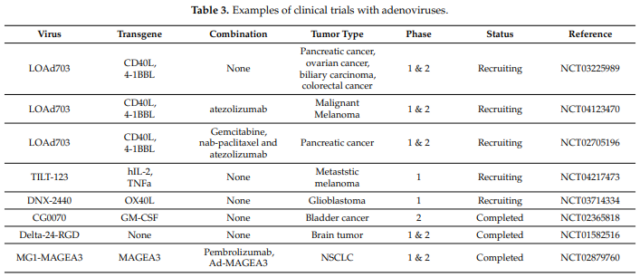
01) H101(Oncorine)
H101 (Oncorine): Recombinant human type 5 adenovirus with deletion of E1B region. The China Food and Drug Administration approved it for the treatment of nasopharyngeal carcinoma in 2005.
02) DNX-2401(Delta-24-RGD)
DNX-2401 (Delta-24-RGD): E1A region deletion 24bp needs to replicate in Rb mutant tumor cells, virus capsid fibers insert arg-gly-asp (RGD) sequence, which can target integrins on cancer cells Receptor. At present, there are a number of trials in combination drugs that have completed phase I trials, and phase II trials in combination with pembrolizumab monoclonal antibody are in progress.
Clinical trial subjects: brain tumor/glioma patients, ovarian cancer and primary peritoneal cancer patients
03) Enadenotucirev
Enadenotucirev: a chimera of adenovirus type 11 and adenovirus type 3. The virus is a chimeric virus obtained by mixing 7 adenoviruses of different serotypes to infect human colorectal cancer cells HT-29, and then isolated from them. Preclinical studies have shown that its replication ability and tumor killing ability are significantly better than ONYX-015. At present, 3 clinical Phase I trials have been completed.
Clinical trial subjects: patients with advanced epithelial tumors
Monotherapy Stage I: 61 patients with advanced epithelial tumors received intravenous infusion of up to 10^13 viral particles. The third-grade side effects were hypoxia, lymphopenia and neutropenia.
Combination therapy phase I trial recruitment: ①Combined use with nivolumab (PD-1 inhibitor) ②Combined radiotherapy and chemotherapy (capecitabine)
04) LOAd703
LOAd703: encoding immunostimulatory protein: trimeric CD40 ligand and 4-1BB ligand.
Monotherapy phase I recruiting: patients with pancreatic cancer, ovarian cancer, biliary tract cancer, and colorectal cancer
Combination therapy phase I and phase II recruitment: ①LOAd703 combined with checkpoint inhibitor atezolizumab in the treatment of patients with malignant melanoma. ②LOAd703 combined with gemcitabine, paclitaxel and checkpoint inhibitor atezolizumab in the treatment of patients with pancreatic cancer.
05) ONCOS-102(CGTG-102
ONCOS-102 (CGTG-102): A chimera of adenovirus types 3 and 5, carrying the cytokine GM-CSF.
Clinical trial subjects: patients with advanced solid tumors
Combination therapy stage I: 12 patients with advanced solid tumors that did not respond to existing treatments were injected with 3E+10^10-3E+10^11PFU virus intratumorally, combined with low-dose cyclophosphamide therapy. The results showed that the pro-inflammatory cytokines of the combined treatment patients increased in a short time, and the tumor lymphocyte infiltration increased.
04. Reovirus
Reovirus: non-enveloped double-stranded, segmented RNA virus, isometric icosahedron, often spherical in appearance, without envelope, virus capsid is composed of 1 to 3 layers of protein shell, diameter Between 60~80nm.
The viral genome is linear dsRNA, with a total of 10-12 RNA fragments (the number of fragments varies between genera), the relative molecular mass of a single RNA is between 0.2×10^6~3.0×10^6, and the total length of the genome is 18200~30500nt. The relative molecular mass is 12×10^6~20×10^6, and nucleic acid accounts for about 15%-20% of the weight of virus particles.
The 5’end of each double-stranded sense strand consists of a methylated nucleotide cap structure (m^7 G^5′ GmpNp), and there is a phosphorylated end on the negative sense strand, and both strands have 3′ -OH, and the virus mRNA lacks the 3’end Poly(A) tail.
The clinical trial information is summarized as follows: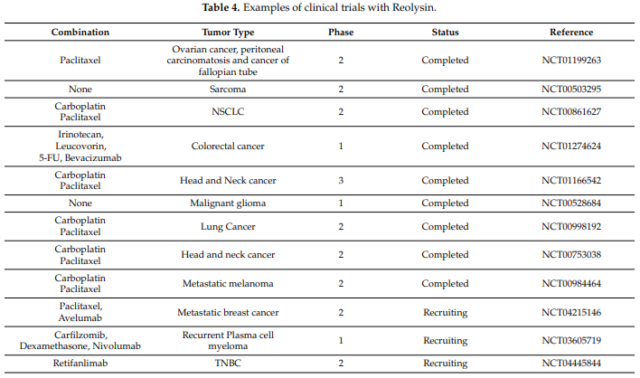
Due to the overexpression of the oncogene Ras and the damage of the type I interferon (IFN) signaling pathway, wild-type reovirus can replicate in cancer cells preferentially, and the oncolytic effect is significant. Reolysin (Pelareorep), an unmodified reovirus type 3, Dearing strain, has completed clinical phase III trials.
Clinical trial subjects:
patients with metastatic breast cancer, advanced head and neck cancer, metastatic ovarian cancer, malignant glioma, prostate cancer and metastatic melanoma
Monotherapy Phase I:
①19 patients with advanced solid tumors (breast cancer, head and neck cancer and melanoma) were injected with 1E+10^10 PFU virus into the tumor. The treatment was well tolerated, and the side effects were grade 2 local erythema and transient flu-like symptoms. Of the 19 patients, 1 had a complete response, 2 had a partial response, and 4 had a stable condition.
②33 patients with advanced cancer received intravenous injection of 3E+10^10 TCID50 virus with Reolysin. Side effects are fever, fatigue and headache. Evidence of virus localization and replication in tumors was observed in 3 patients, and no anti-tumor efficacy was observed.
③18 patients with advanced solid tumors received intravenous injection of Reolysin1E+10^8-3E+10^9 TCID50 virus every 4 weeks. Side effects are fatigue and fever. The overall clinical benefit rate was 45%: 1 patient had a partial remission, and 7 patients were in stable condition.
Monotherapy Phase II:
① In 21 patients with metastatic melanoma, Reolysin was injected intravenously with 3E+10^10 TCID50 virus. A large amount of reovirus replication was observed in tumor biopsy of 2 patients, but no objective reaction was observed.
②43 patients with bone and soft tissue sarcoma metastasized to the lungs received intravenous injection of Reolysin every 28 days. Side effects are mild to moderate, including fever, chills, fatigue, diarrhea, cough, dyspnea, neutropenia, and thrombocytopenia. 14 cases were stable for more than 2 months, and 5 cases were stable for more than 6 months.
Combination therapy phase I:
①Reolysin combined with chemotherapy to treat 23 cases of different advanced solid tumors. In the first stage, the patient received 20Gy radiation and two intratumoral injections of 1E+10^8-1E+10^10TCID50 virus.
In the second stage, the patient received 36Gy radiation, 2, 4 or 6 doses of 1E+10^10 TCID50 virus. Of the 7 evaluable patients in the low-dose radiation group, 2 showed partial responses, and 5 patients were in stable condition. In the high-dose radiotherapy, 12 had partial response and 2 had a stable condition.
②Reolysin combined with gemcitabine in the treatment of 16 patients with advanced cancer, received a single dose of gemcitabine and 3E+10^10TCID50 reovirus injection. Three of the 16 patients had dose-limiting toxicity, two patients had liver enzyme levels raised to grade 3, and one patient had troponin levels raised to grade 3. In this combination therapy, the neutralizing antibody response to reovirus was delayed and weakened.
Combination therapy in Phase II and III trials: combined with carboplatin, irinotecan, cyclophosphamide, paclitaxel and docetaxel.
05. Outlook
As a new tumor treatment method, oncolytic virus is safe tolerated and has obvious anti-tumor efficacy. According to a number of clinical data, combination therapy can achieve better therapeutic effects than single-agent therapy. Because oncolytic viruses may destroy the immunosuppressive microenvironment in tumors, recent clinical trials have focused on their combination with immunotherapy, especially with immune checkpoint inhibitors.
Looking forward to the field of oncolytic viruses in the future, advances in virus manufacturing technology may increase its anti-tumor efficacy; intravenous injection of oncolytic viruses may gradually replace intratumoral administration. More oncolytic virus drugs will soon be approved by regulatory agencies for the treatment of different malignancies.
(source:internet, reference only)
Disclaimer of medicaltrend.org



