How to evaluate the vaccine against COVID-19 variants like Delta Lambda..?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
How to evaluate the vaccine against COVID-19 variants like Delta Lambda..?
How to evaluate the vaccine against COVID-19 variants like Delta Lambda..? Delta and Lambda mutant strains are coming fiercely! How to evaluate the protection of COVID-19 vaccine against Delta and Lambda variants?
On August 15, there were 197,340 deaths from Peru’s COVID-19 pneumonia. Currently, more than 80% of the new COVID-19 cases are caused by the Lambda mutant strain. The per capita death rate of COVID-19 has soared to the world’s first.

Delta mutant
At the end of 2020, the B.1.617 variant appeared for the first time in India and spread throughout India and at least 90 countries. It has now become the main strain in many regions of the world. B.1.617 contains three sub-lines B.1.617.1 (Kappa), B.1.617.2 (Delta) and B.1.617.3.
Documents from the CDC in the United States show that the R0 of the original new coronavirus is 2.6 (1.5-3.5), which means that a new coronavirus patient will infect other 2.6 people on average; and the current Delta mutant R0 has reached 5-9.5, with an average of 7.5, which is one infection People who have the Delta mutant strain may further infect 7.5 other people, and the transmission effect is similar to chickenpox.
Delta strains have mutations of T19R, G142D, Δ156-157, R158G, L452R, T478K, D614G, P681R, D950N. The mutations of Kappa strain include T95I, G142D, E154K, L452R, E484Q, D614G, P681R, Q1071H. Both Kappa and Delta mutant strains carry L452R mutation, D614G mutation, and P681R mutation. Unlike Kappa, the Delta mutant does not have E484Q, but has a T478K mutation.
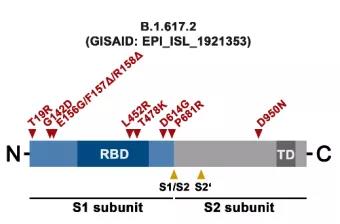
The mutation site of the Beta mutant (from the Internet)
Why are Delta mutants so fierce?
First, the multiple mutation sites of the Delta mutant strain enhance infectivity. Its RBD domain contains L452R and T478K mutations. The L452R mutation reduces antibody-mediated neutralization. T478K is speculated to increase infectivity; the D614G mutation is located between the RBD and S1/S2 cleavage sites, which enhances binding to ACE2 and causes infection. Increased sex; P681R may increase the cleavage of S protein S1/S2 sites and increase the infectivity of virus particles.
Secondly, the Delta mutant strain has stronger replication ability and higher viral load. The research team of Lu Jing, an epidemiologist at the Guangdong Provincial Center for Disease Control and Prevention, reported in a preprinted paper published on July 12 that the Delta strain can be detected 4 days after exposure, while the original strain can be detected after exposure. The average detection time is 6 days, which shows that the Delta strain replicates much faster. The viral load of those infected with the Delta strain is also up to 1260 times higher than those infected with the original strain.
Furthermore, the Delta mutant strain can evade the neutralizing ability of neutralizing antibodies. Moderna announced its mRNA new coronavirus vaccine mRNA-1273, a research article “Serum Neutralizing Activity of mRNA-1273 against SARS-CoV-2 Variants” on the neutralizing ability of a variety of new coronavirus mutant strains including Delta. The results of the study showed that compared with the wild-type new coronavirus strain containing the D614G mutation, the neutralizing titer of the Delta virus strain’s serum was reduced by 2.1 times. A study released by Johnson & Johnson also showed that the serum of subjects vaccinated with Ad26.COV2.S adenovirus vaccine had a 1.6-fold reduction in the ability to neutralize Delta variants compared with the control group.
Finally, the pathogenicity of those infected with the Delta mutant strain increases. The clinical manifestations of Delta virus are no different from the symptoms caused by previous strains, but the proportion of people with symptoms among the infected has increased.
Lambda mutant
In August 2020, Peru was the first to report a case of Lambda variant infection (Pango is C.37, GISAID clade number is GR/452Q.V1). When the medical community first noticed Lambda, only 1 case could be detected from 200 samples. By March 2021, about 50% of the COVID-19 cases in Lima, the capital of Peru, originated from this variant. WHO data shows that between May and June this year, 82% of new cases in Peru were caused by the Lambda variant, and the per capita death rate of COVID-19 soared to the highest in the world. At present, the mutant strain has spread in dozens of South American countries including Peru, Chile, Argentina, and Ecuador. In the United Kingdom and other countries, infections with the Lambda mutant strain have also been found.
Lambda mutants have many mutations in the spike protein, mainly including G75V, T76I, del247/253, L452Q, F490S, D614G and T859N.
Brazilian researchers published studies reporting that this variant strain may cause more gastrointestinal (diarrhea) symptoms. Judging from the genome sequence, the Lambda mutant strain lacks the gene fragment Δ246-252, which may enhance its adaptability to the infected host. At the same time, the mutations of L452Q and F490S are located in the virus receptor binding region (RBD). The L452Q variant is similar to the L452R variant, which can be found in the Delta variant. Studies have confirmed that this mutation can increase the ability of the virus to invade cells, that is, enhance infectivity. The F490S variant will reduce the neutralizing ability of the antibody.
Researchers from the University of Tokyo published a paper in the preprint bioRxiv, revealing the evolutionary characteristics of the Lambda mutant strain, pointing out that the Lambda mutant strain is more infectious and immune.
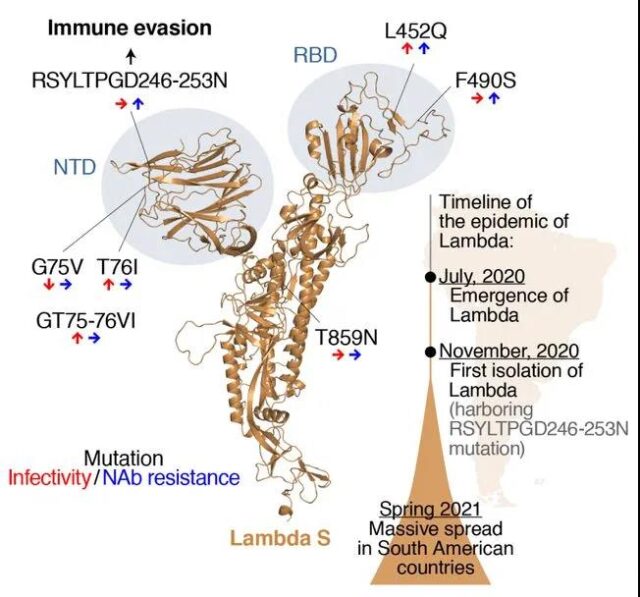
The mutation site of Lambda mutant strain (from: SARS-CoV-2 Lambda variant exhibits higher infectivity and immune resistance)
The study found that the RSYLTPGD246-253N, L452Q and F490S mutations of the Lambda mutant strain lead to increased immune resistance; and the T76I and L452Q mutations lead to increased infectivity. This allows the Lambda strain to have the ability to increase infectivity and immune escape at the same time, thus gaining the potential for large-scale spread.
Are current COVID-19 vaccines effective against mutant strains?
On April 6, 2021, the “Susceptibility of Circulating SARS-CoV-2 Variants to Neutralization” article was published in the New England Journal of Medicine. The Beijing Institute of Microbiology and Epidemiology, the Chinese Center for Disease Control and Prevention and other institutions used SARS-CoV- 2Pseudovirus system to verify whether the neutralizing antibodies in the serum of vaccinators who have been vaccinated with BBIBP-CorV or CoronaVac can resist the new coronavirus mutant. Pseudoviruses include Wuhan type 1 (wild type), D614G mutant strain, Alpha (B.1.1.7) and Beta (B.1.351) mutant strains.
The results of the study showed that the Alpha mutant strain had almost no resistance to the neutralizing activity of the convalescent serum or the vaccinated sera, while the Beta mutant strain had more neutralizing activity against the convalescent sera and the vaccinated sera than the wild-type virus. resistance.
On May 20, the New England Journal of Medicine published an article on the protection rate of the Covid-19 vaccine against the Beta (South African strain, B.1.351) mutant strain: “Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B. 1.351 Variant”, the article announced the effects of two vaccines on the South African mutant B.1.351, namely ChAdOx1 nCoV-19 (Oxford-AZ, adenovirus vector vaccine) and NVX-CoV2373 (Novavax-CEPI, recombinant protein vaccine).
The results show that the ChAdOx1 nCoV-19 vaccine has no effective protection against mild to moderate symptomatic infection of the South African variant strain. The specific data are as follows
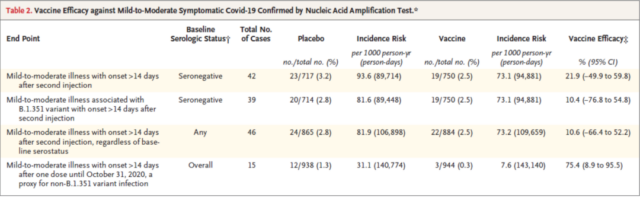
ChAdOx1 vaccine protection efficiency against mutant strains (from: Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant)
The recombinant protein vaccine NVX-CoV2373 has not yet entered clinical phase III. Data results show that among HIV-negative and baseline plasma antibody-negative populations, the vaccine’s protective efficacy against symptomatic COVID-19 infection is 60.1%, and it is HIV-negative and baseline plasma antibody Among those who are positive, the effective protection of the vaccine against symptomatic COVID-19 infection is 52.2%, which is a decline.
On July 21, the “New England Journal of Medicine” published a report “Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant”. The research focuses on the effectiveness of BNT162b2 (Pfizer-BioNTech, mRNA vaccine) and ChAdOx1 nCoV-19 (Oxford-AZ, adenovirus vector vaccine) against the Delta mutant strain. The results show that two doses of BNT162b2 or ChAdOx1 nCoV-19 COVID-19 vaccine The effect on the Delta mutant strain is almost as effective as the previously dominant Alpha mutant strain.
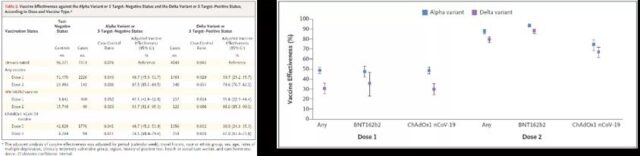
Protection rate of BNT162 and ChAdOx1 vaccines against mutant strains (from: Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant)
In an interview, Yang Xiaoming, chairman of CHINA SINOPHARM China Biotech, said that the inactivated vaccine of Sinopharm can achieve cross-neutralization of four typical mutant strains (including the Delta strain) in the experiment, and can provide effective protection. In addition, the Sri Lankan research institute issued a real-world protective power research report on the effectiveness of Sinopharm vaccines on July 20. The study showed that Sinopharm vaccines have formed immunity to the currently circulating variants of Delta and Beta strains. Compared with the new coronavirus strain that first circulated in China, the antibody titer of the vaccinators against the Delta strain was only 1.38 times lower. The Chinese biological vaccine still has a better protective effect on the Delta strain. Sinopharm China Biotech’s inactivated vaccine against the Delta variant strain is also under development.
At the same time, the person in charge of Kexing Biology also stated that Kexing Biology has obtained samples of multiple epidemic variants around the world, and the neutralizing antibody experiments conducted on different variants have seen significant results. Chile, which uses Coxing vaccine, recently published real-world protection data, showing that in areas where the new coronavirus Gamma strain is endemic, Coxing vaccine has obvious protective effects, and the study of serum neutralizing antibodies of the Delta strain has also seen Similar results for the Gamma strain. The Delta strain is currently being used for the development and production of new vaccines, and this research will be further promoted next.
Judging from the disclosed data, some vaccines have a good protective effect on mutant strains, while some mutant strains have higher resistance to the neutralizing antibodies produced by the vaccine (that is, the vaccine is easily ineffective). Some vaccines are ineffective against certain mutant strains, such as ChAdOx1 nCoV-19 against Beta mutant strains.
How to evaluate vaccine potency?
From real-world data, we can see that not all vaccines have a good protective effect on mutant strains, and in principle, vaccines will not achieve 100% protection, and individual immune responses must be different. Therefore, it is necessary to evaluate the valence of the protection rate of the mutant strain of the vaccine to guide vaccination, epidemic prevention and control, and disease treatment.
One way to evaluate vaccine potency is to use real-world data to count the population’s resistance to mutant strains after vaccination, so that the most realistic data can be obtained. However, it is costly and time-consuming, and it may not be possible to accurately count the impact of a single mutant strain. It is still affected by multiple factors, which is not conducive to quickly drawing guiding opinions. And if the vaccine is ineffective or the protection rate is low, the vaccinated population will be at high risk.
Therefore, clinical and experimental methods are often used in vaccine evaluation.
In the experiment, the mutant strain challenge experiment after the animal is injected with the vaccine can be used to statistically verify the effectiveness of the vaccine. However, it is expensive, time-consuming, and there is still a gap between animals and humans (human body is against ethics), and the data obtained need other experiments to corroborate.
In order to further verify the effectiveness of the vaccine, the serum of the vaccinators will be drawn clinically, Elispot will be used to detect the content of memory B cells (whether cellular immunity is activated), and the neutralizing antibody titers contained therein (whether humoral immunity is activated) ), and the neutralizing effect of neutralizing antibodies on virus mutant strains.
Pseudovirus system and immune blocking methods are commonly used to evaluate vaccine-induced neutralizing antibodies.
Fake virus system
Neutralizing antibodies can prevent viruses from infecting cells. Add neutralizing antibodies (serum/neutralizing antibody positive control after vaccine injection) to the virus-infected cell system to detect the condition of the infected cells and calculate the blocking effect.
Due to the high infectivity and pathogenicity of the new coronavirus, the use of live new coronavirus for infection verification must be carried out in a biosafety level 3 or above laboratory, which is limited by laboratory conditions and the source of the virus. In addition, due to the different strains, culture conditions, and evaluation criteria of the results, there are often some differences in the results of live virus detection in different laboratories. In terms of safety and operability, it is more convenient to use pseudoviruses, and after pseudoviruses infect cells, fluorescence readings can be used for quantification, which is easy to achieve standardization, which is beneficial to assess the antiviral effects of vaccines in preclinical and clinical stages.
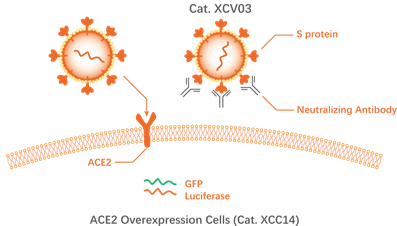
Neutralizing antibodies inhibit COVID-19 pseudo-disease
Pseudovirus detection system: New coronavirus pseudovirus (Novoprotein Cat.XCV01-XCV11) has no self-replication ability, and its surface expresses S protein (including various mutants), and contains green fluorescent protein (GFP) or luciferase (Luciferase) reports Gene, can specifically infect cells expressing ACE-2 (Novoprotein Cat.XCC14). Cells infected by the virus express GFP or Luciferase, which can be detected by a microplate reader for quantitative analysis. Pseudovirus assays can achieve high-throughput detection and standardized detection. By replacing the mutant S protein, the cross-neutralization effect of neutralizing antibodies against different mutant strains can be studied.
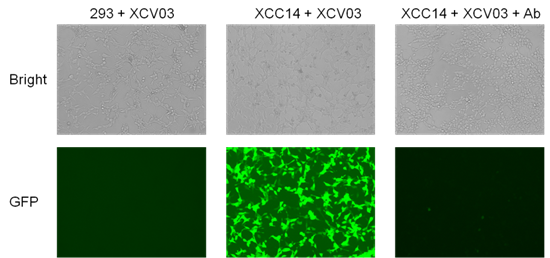
Neutralizing antibody inhibits pseudovirus infecting cells, flow cytometric detection of fluorescence positive rate and MFI value, fluorescence inverted microscope to take fluorescence photos of cells after infection; 293 cells that do not express ACE-2 are not infected
Using the pseudovirus system, Nearshore Protein verified the blocking effect of a neutralizing antibody against 9 new coronavirus mutant strains:
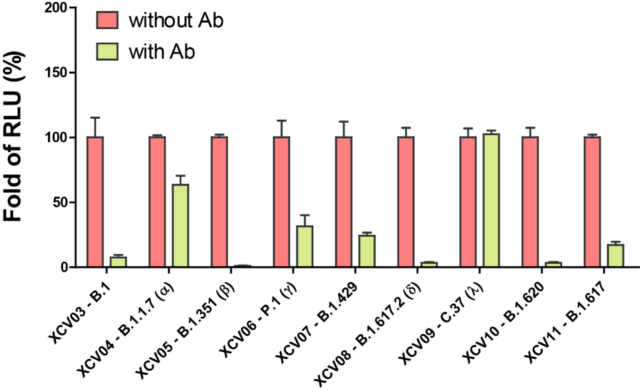
Neutralizing antibodies inhibit pseudovirus infection of cells
The results show that this neutralizing antibody has a very good blocking effect on B.1, B.1.351 (Beta), B.1.617.2 (Delta), and B.1.620 mutant strains, and has a good blocking effect on P.1 (Gamma), B.1.429 The blocking effect of B.1.617 (Kappa) mutant strain is average, and it has almost no blocking effect on B.1.1.7 (Alpha) and C.37 (Lambda) mutant strains. This data confirms the situation reported in the literature that the protective effects of vaccines or neutralizing antibodies in the serum of recovered patients are very different before different mutant strains.
Immune blocking verification
For people after large-scale vaccination, to quickly evaluate the protective effect of neutralizing antibodies for each person, a simple and fast immune blocking verification method can be used.
Immune blocking verification uses the principle that neutralizing antibodies block the binding of S-RBD protein to ACE-2. After adding neutralizing antibodies, detecting the reduced amount of S-RBD protein bound to ACE-2 is used to calculate the drop of neutralizing antibodies. Spend.
Drop the vaccinated serum into the sample area on the left. The serum chromatograms to the right under capillary action, and drives the fluorescent nanosphere labeled S-RBD (Novoprotein Cat.DRA72) on the binding pad to move together. After the serum reaches the detection area, S-RBD binds to the ACE-2 (Novoprotein Cat.C05Y) immobilized on the detection line and is fixed on the detection line, and the neutralizing antibody in the serum competitively inhibits S-RBD and ACE -2 combined to reduce the S-RBD fixed on the detection line. Through the intensity and ratio of the fluorescence intensity of the detection line and the quality control line, the fluorescence change can be accurately and quantitatively detected, so as to standardize the calculation of the neutralizing antibody inhibitory effect.
Nearshore protein provides a series of COVID-19 mutant pseudoviruses including Delta mutant strain (B.1.617.2) and Lambda mutant strain (C.37), as well as the corresponding S protein and ACE2 protein.
How to evaluate the vaccine against COVID-19 variants like Delta Lambda..?
How to evaluate the vaccine against COVID-19 variants like Delta Lambda..?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



