AstraZeneca Imfinzi+tremelimumab+chemotherapy for lung cancer!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
AstraZeneca Imfinzi+tremelimumab+chemotherapy for stage 4 lung cancer!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
AstraZeneca Imfinzi+tremelimumab+chemotherapy for stage 4 lung cancer!
First-line immunotherapy for stage 4 lung cancer! AstraZeneca’s Imfinzi+tremelimumab+ chemotherapy regimen: Significantly delay disease progression and prolong life!
Compared with chemotherapy, the triple regimen extended overall survival (OS) by 23% and disease progression-free survival (PFS) by 28%.
AstraZenecarecently in 2021 the World Conference on Lung Cancer (WCLC2021) announced positive results on three POSEIDON study. The study evaluated the anti-PD-L1 therapy Imfinzi (Infinzi, generic name: durvalumab, durvalumab) combined with anti-CTLA-4 therapy tremelimumab and chemotherapy, chemotherapy alone as the first-line treatment of stage IV (metastatic) non-small Efficacy and safety of patients with cell lung cancer (NSCLC) .
The results showed that compared with patients receiving chemotherapy, patients receiving Imfinzi+tremelimumab+ chemotherapy showed a statistically significant and clinically significant improvement in overall survival (OS) and progression-free survival (PFS) (28% improvement in PFS) , OS improved by 23%). The results show that adding a short course of tremelimumab to the Imfinzi+ chemotherapy regimen can improve the prognosis of patients without increasing treatment interruption.
Susan Galbraith, Executive Vice President of Oncology Research and Development of AstraZeneca , said: “POSEIDON research data has enabled patients to further benefit from Imfinzi. This is an important verification of our exploration of the new combination development strategy.
For patients who have already received chemotherapy, a short course of treatment is added to Imfinzi. Tremelimumab, compared with chemotherapy alone, reduces the risk of cancer progression or death by 28%. The results of the study also show that the significant improvement in survival rate in the first-line treatment of patients with metastatic non-small cell lung cancer does not affect tolerance. We Looking forward to discussing these data with regulators.”
POSEIDON is a randomized, open-label, multi-center, global phase 3 clinical study that evaluates the efficacy and safety of Imfinzi+platinum-containing chemotherapy, Imfinzi+tremelimumab+chemotherapy, and chemotherapy in the first-line treatment of patients with metastatic NSCLC. The study included patients with non-squamous and squamous diseases and full range of PD-L1 expression levels, and excluded patients with EGFR or ALK gene mutations.
The results showed that during 16 weeks, compared with a series of chemotherapy regimens, patients who received Imfinzi+ chemotherapy and a short course of 5 cycles of tremelimumab had a 23% lower risk of death (HR=0.77; 95%CI: 0.65-0.92; p =0.00304). The median OS of patients in the combination treatment group was 14.0 months, while that of the chemotherapy group was 11.7 months. After 2 years, an estimated 33% of patients in the combination therapy group were alive, compared with 22% in the chemotherapy group.
In addition, compared with chemotherapy, Imfinzi+chemotherapy+tremelimumab treatment also reduced the risk of disease progression or death by 28% (HR=0.72; 95%CI: 0.60-0.86; p=0.00031). The median PFS of patients in the combination therapy group was 6.2 months, compared with 4.8 months in the chemotherapy group. The safety of Imfinzi+chemotherapy+tremelimumab regimen is about the same as that of Imfinzi+chemotherapy regimen, and it did not lead to an increase in treatment interruption.
The POSEIDON study also tested the effect of Imfinzi+ chemotherapy. The results showed that compared with chemotherapy, patients receiving Imfinzi+ chemotherapy had a statistically significant improvement in PFS (HR=0.74; 95%CI: 0.62-0.89; p=0.00093). The positive OS trend observed with Imfinzi+ chemotherapy did not reach statistical significance.
In this study, the safety of each Imfinzi combination regimen was consistent with the known safety of a single drug, and no new safety signals were found.
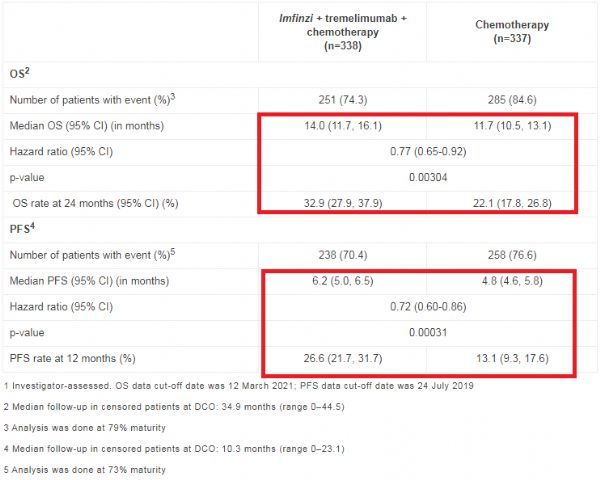
POSEIDON research results
Lung cancer is the leading cause of cancer deaths in men and women, accounting for about one-fifth of all cancer deaths. Lung cancer is roughly divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), of which NSCLC accounts for about 80-85%. In NSCLC, patients are classified as squamous cell carcinoma (approximately 25-30%) or non-squamous cell carcinoma (approximately 70-75%).
Stage 4 lung cancer is the most advanced type of lung cancer and is often referred to as metastatic disease. Most patients have cancer that has spread beyond the lungs at the time of diagnosis. For patients with metastatic disease, the prognosis is very poor, and the 5-year survival rate after diagnosis is only 10%.
Imfinzi is a human monoclonal antibody that binds to programmed cell death factor ligand 1 (PD-L1), blocks the interaction of PD-L1 with PD-1 and CD80, resists tumor immune evasion strategies and releases immunity Inhibition of the reaction.
Based on the results of the Phase 3 PACIFIC study, Imfinzi is the only immunotherapy approved for curative treatment in patients with unresectable locally advanced (phase III) NSCLC after chemotherapy and radiotherapy, and has now become the global standard of care . In addition, based on the results of the Phase 3 CASPIAN study, Imfinzi has also been approved for the treatment of extensive-stage small cell lung cancer (ES-SCLC) in many countries around the world.
Currently, AstraZeneca is conducting multiple registration trials, focusing on evaluating Imfinzi for the treatment of early lung cancer, including potential cures (PACIFIC-2, -4, -5, MERMAID-1, -2, AEGEAN, ADJUVANT BR.31, ADRIATIC and other phase 3 trials). In addition, the company is also conducting two phase II platform trials in the phase III unresectable phase (COAST) and neoadjuvant early treatment phase (NeoCOAST) to test Imfinzi’s new combination therapy.
AstraZeneca Imfinzi+tremelimumab+chemotherapy for lung cancer!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



