Can Probiotics prevent Alzheimer’s disease as regulator of brain function?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Can Probiotics prevent Alzheimer’s disease as the regulator of brain function?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Can Probiotics prevent Alzheimer’s disease as the regulator of brain function?
Alzheimer’s disease mostly occurs in pre-senile and old age, and those who develop after 65 years of age are called Alzheimer’s disease (Alzheimer’s disease, AD). At present, about 50 million people worldwide are plagued by dementia, which has become an important health problem worldwide.
Some people say that Alzheimer’s is the most vicious God’s curse. Because the cause of it is unknown, there is no medicine to treat the cause, which can only delay the course of the disease. Although a variety of methods for the treatment of AD have been studied before, none of them have shown the effect of improving the disease. This may be due to the poor timing of administration during the progression of AD.
Recent clinical studies have shown that two decades before the appearance of the clinical symptoms of AD, there were pathological changes characterized by β-amyloid (Aβ) plaques and fibrous tangles of hyperphosphorylated tau protein. Monoclonal antibodies that selectively target protein aggregation for early intervention have shown good therapeutic potential. This suggests that early diagnosis and intervention for AD and mild cognitive impairment (Mild cognitive impairment, MCI) relief is necessary, and has a positive meaning.
More and more studies have shown that the intestinal microbiota is one of the key regulators of intestinal brain function. The microbiota and the brain communicate with each other through various channels, including the immune system, the vagus nerve, and the enteric nervous system (that is, the microbiota-gut-brain axis system). Therefore, by exploring the relationship between the microbiota-gut-brain axis and finding a targeted microbiota that maintains brain health, it has become a promising disease intervention.
Recently, the team of Takuji Yamada from Tokyo Institute of Technology in Japan published a research paper titled Identification of Faecalibacterium prausnitzii strains for gut microbiome-based intervention in Alzheimer’s disease-type dementia in Cell Reports Medicine. Comparative healthy, mild faeces 16S rRNA gene sequencing cognitive dysfunction (Mild cognitive impairment, MCI) and Alzheimer’s disease (Alzheimer’s disease, AD) group consisting intestinal microflora found gut critical strain – P Faecalibacterium prausnitzii (F. prausnitzii) has the effect of protecting people from suffering from dementia, and emphasizes that F. prausnitzii strain can be used as a gut-based microbiological means to intervene in the potential population of AD-type dementia.
Research contents
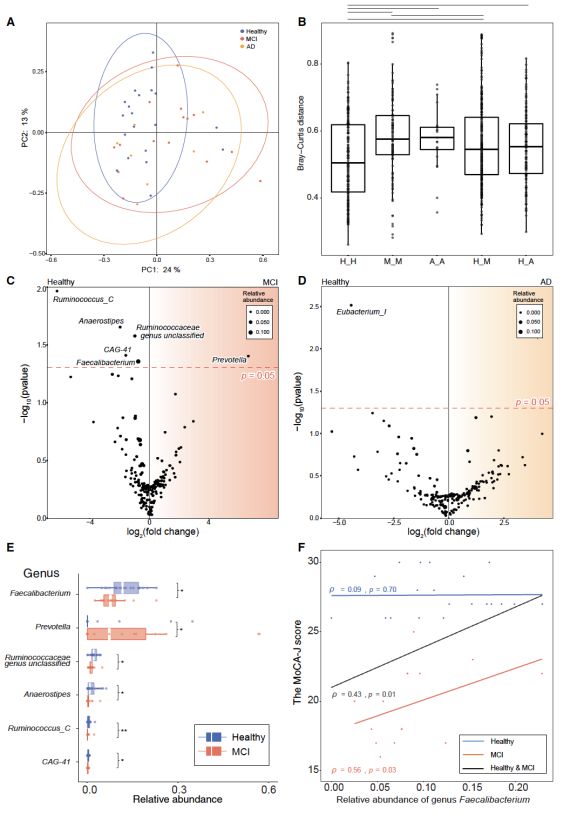
First, in order to determine the potential microbial populations to prevent MCI, the authors conducted a cross-sectional study. The author collected human fecal samples from the healthy group, the MCI group, and the AD group in Kusatsu, Gunma Prefecture, Japan. The composition of intestinal microbes was analyzed by 16S rRNA gene sequencing, and a total of 129 genera were detected. After that, the author used Bray-Curtis PCoA and permutation multivariate analysis of variance (PERMANOVA) to detect the composition profile of the overall genera.
The results showed that there was a difference in microbial genus composition between the healthy group and the MCI group (R2 = 0.0465, p = 0.0968), but there was no difference between the healthy group and the AD group (R2 = 0.0534, p = 0.1423). Among them, the distance between MCI and AD group was significantly higher than that of healthy group. There was no difference in the Shannon-Wiener Alpha Diversity Index between the groups.
Among 129 genera, the differential abundance analysis of ALDEx2 showed that the abundance of 6 genera was significantly different between the healthy group and the MCI group. Compared with the healthy group, the abundance of Faecalibacterium, Ruminococcaceae, Anaerostipes, Ruminoccocus_C and CAG-41 in the MCI group was significantly reduced, and the abundance of Prevotella in the MCI group was significantly increased. The abundance of Eubacterium differed significantly between the healthy group and the AD group.
Furthermore, the authors found that the abundance of Faecalibacterium prausnitzii was most significantly correlated with MCI disease, and the abundance of this strain was significantly decreased in the MCI group.
Based on the comparative analysis of intestinal microbial composition, the author further selected and isolated the F. prausnitzii strain as the most promising candidate for the prevention of mild cognitive impairment .
In order to examine the causal relationship between F. prausnitzii and cognitive ability, the author used the isolated F. prausnitzii strain in an AD mouse model, and successfully identified two of the effective strains, Fp14 and Fp360.
The Aβ-injected mice were treated with Fp14 and Fp360 orally, and the results showed that live Fp360 can significantly improve Aβ-induced cognitive impairment in the Y maze test and passive avoidance test, but Fp360 after pasteurization has no such effect . Interestingly, in Fp14-treated mice, Fp14 after pasteurization can significantly improve Aβ-induced cognitive impairment.
After confirming that Fp14 and Fp360 are effective strains, the specific orthologs found only in these effective strains were determined by whole-genome comparison and metagenomic shotgun sequencing, and the healthy group was more abundant than the MCI group. In addition, metabolome and RNA sequencing (RNA-seq) analysis showed that there is a relationship between Fp14 and host oxidative stress and mitochondrial function in the brain.
Since one of the limitations of F. prausnitzii is its high sensitivity to oxygen, we further explored whether the inactivated Fp14 obtained after pasteurization has the effect of relieving cognitive impairment in the brain. Therefore, the author further analyzed the Fp14 after pasteurization, and performed metabolomics and RNA-seq analysis on the hippocampus obtained from behavioral animal experiments to explore the potential therapeutic mechanism.
Metabolome analysis detected 355 metabolites. Among them, Fp14 after pasteurization significantly reduces the content of thymine and N6-methyl-2′-deoxyadenosine, and suberic acid also tends to decrease. It has been reported that these metabolites are related to oxidative stress and mitochondrial function.
RNA-seq analysis also detected 15 differentially expressed transcripts. Among them, Fp14 after pasteurization significantly reduces the transcription level of the protein PACS-2, which is also reported to be related to mitochondrial function.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



