Johnson & Johnson vaccine program safely induces a strong immune response
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The Johnson & Johnson vaccine program (Zabdeno®, Mvabea®) safely induces a strong immune response in healthy adults and HIV adults!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The Johnson & Johnson vaccine program (Zabdeno®, Mvabea®) safely induces a strong immune response in healthy adults and HIV adults!
Recently, data from a study published in the journal PLOS Medicine in the United States showed that Johnson & Johnson (JNJ)’s Ebola vaccine program (2-dose immunization)-Zabdeno® (Ad26.ZEBOV) And Mvabea® (MVA-BN-Filo) are well tolerated in healthy adults and HIV-infected adults, and induce a strong immune response.
These findings, together with the recent Phase 3 clinical data published in the “Lancet Infectious Diseases”, support the potential preventive use of this vaccine program to protect people at risk of Ebola (Ebola) crowd.
Johnson & Johnson’s Ebola vaccine program (Zabdeno®+Mvabea®) received marketing authorization from the European Commission (EC) in July 2020, and was prequalified by the World Health Organization (WHO) in April 2021.
Since the outbreak of the West Africa epidemic in 2014, with the power of global public-private cooperation, Johnson & Johnson’s Ebola vaccine program (Zabdeno®+Mvabea®) obtained regulatory approval in less than 6 years.
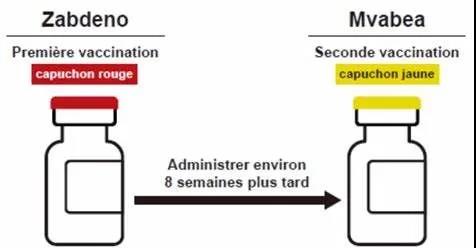
The specific vaccination plan of this vaccine program is:
(1) Zabdeno® is used as the first immunization vaccine, which is developed based on Janssen’s AdVac technology;
(2) After 2 months (approximately 8 weeks), Mvabea® is used as the second immunization vaccine. Needle immunization vaccine, the vaccine is based on Bavarian Nordic’s MVA-BN technology.
The data published in the journal PLOS Medicine come from the second phase of the EBL2002 study. The study was conducted in Burkina Faso, Côte d’Ivoire, Kenya and Uganda and enrolled 668 healthy adults and 142 HIV-infected adults. During the study, no worrying safety signals were found.
Paul Stoffels, MD, Vice Chairman and Chief Scientific Officer of Johnson & Johnson’s Executive Committee, said: “These data add more and more evidence to support the preventive use of Johnson & Johnson’s Ebola vaccine program (Zabdeno®+Mvabea®) to protect people with Ebola infections.
This is critical to our vision of protecting the world’s most vulnerable and underserved people (including those living with HIV) by preventing an Ebola outbreak before the Ebola outbreak.
Recently, the Ebola virus The re-emergence of the Democratic Republic of the Congo is the latest in a series of outbreaks in Africa last year, which further illustrates the need to take a proactive approach to address this continuing public health threat.”
Since the Ebola virus was discovered in 1976, more than 30 outbreaks have been announced, the most serious of which has occurred in Africa in the past seven years. Johnson & Johnson accelerated the development of an Ebola vaccine at the beginning of the 2014-2016 Ebola crisis, which caused more than 11,000 deaths.
Johnson & Johnson responded to the recent Ebola epidemic by providing vaccine programs to governments and the World Health Organization (WHO).
The most recent time was in May 2021, Johnson & Johnson announced that it would provide up to 200,000 doses of Ebola vaccine to support the WHO’s early access to clinical programs in response to the Ebola outbreak in Guinea and the prevention of Ebola in West Africa.
In addition to assisting in the response to the epidemic, the company is also committed to ensuring that the countries and communities most in need in Africa have wider access to its Ebola vaccine program.
Original source: Statement on Data Published in PLOS Medicine on Tolerability and Immune Response of Johnson & Johnson Ebola Vaccine Regimen in Adults Living with HIV
The Johnson & Johnson vaccine program (Zabdeno®, Mvabea®) safely induces a strong immune response in healthy adults and HIV adults!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



