New AVV variant breaks through the blood-brain barrier without being enriched in liver
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New AVV variant breaks through the blood-brain barrier without being enriched in liver
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
New AVV variant breaks through the blood-brain barrier without being enriched in liver.
The blood-brain barrier (BBB) refers to the barrier between blood plasma and brain cells formed by brain capillary walls and glial cells, and the barrier between plasma and cerebrospinal fluid formed by the choroid plexus.
Only specific types of molecules are allowed to pass from the blood. The flow enters the brain neurons and other surrounding cells.
The existence of the blood-brain barrier is of great significance for preventing harmful substances from entering the brain from the blood.
However, the blood-brain barrier also prevents the transfer of most small and macromolecules (such as peptides, proteins and nucleic acids) , which severely restricts the nerves.
Treatment of central system diseases (such as neurodegenerative diseases, brain tumors, brain infections and strokes, etc.) .
With the increasingly serious population aging problem, neurodegenerative diseases such as Alzheimer’s disease, Huntington’s disease, Parkinson’s disease are growing rapidly, and the treatment of brain diseases is facing severe challenges.
Therefore, there is an urgent need to effectively break through the blood-brain barrier. Drug delivery strategy .
Recently, the Viviana Gradinaru team at the California Institute of Technology published a research paper titled: AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset in Nature Neuroscience .
The research developed a new adeno-associated virus (AAV) variant – AAV.CAP-B10 , which can break through the blood-brain barrier through blood injection and target nerve cells without being enriched in the liver , avoiding the possibility The liver toxicity caused by side effects provides a safer and more effective treatment option for brain diseases.
The A the AV variant in mouse and marmoset model have been verified , it is to pave the way for clinical translation.

Adeno-associated virus (AAV) has long been regarded as the most promising gene therapy delivery vector, and it has been clinically verified.
AAV consists of two main parts: a capsid made of protein ; and genetic material wrapped in the capsid .
Replace the genetic material with DNA fragments needed for treatment to form recombinant AAV, and then target it to specific tissues to achieve the purpose of gene therapy.
As early as 2017, the FDA approved the listing of Spark Therapeutics’ AAV gene therapy, which is to deliver the RPE65 gene to the retina through AAV to treat congenital amaurosis.
The Viviana Gradinaru team at the California Institute of Technology has been working on the development and design of AAV capsids that can cross the blood-brain barrier for nearly a decade and enable it to specifically target specific cell types in the brain.
Previously, the team developed AAV-PHP.eB, which can efficiently cross the blood-brain barrier through intravenous injection, but at the same time it will be enriched in the liver.
In the new study, conducted by the team of AAV9 capsid transformation, developed a series of AAV variants, which AAV.CAP-B10 , not only can effectively cross the blood-brain barrier into the brain cells, particularly neurons, while It can also include systemic targets including the liver .
This neuron-specific targeting and low liver targeting across the blood-brain barrier has been confirmed not only in mice, but also in non-human primates, marmosets.
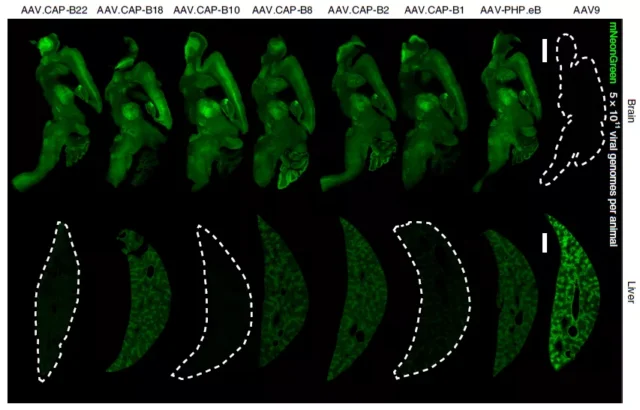
Prior to this, the AAV-PHP.eB and other vectors developed by the team were unsuccessful in non-human primate experiments, and this new study looked for the AAV.CAP-B10 developed in non-human primates.
The same effect is significant in AAV9 and AAV-PHP.eB. Compared with AAV9 and AAV-PHP.eB, AAV.CAP-B10 can specifically target neurons without being enriched in the liver.
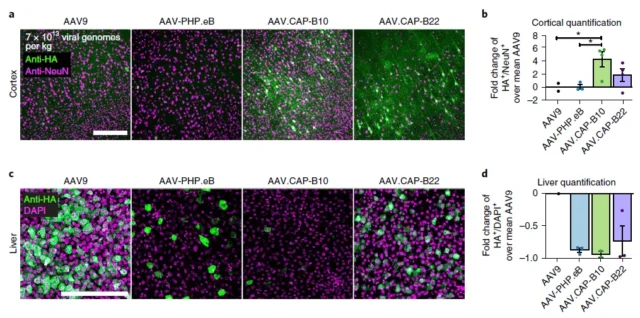
Viviana Gradinaru’s team modified the AAV capsid at multiple sites through directed evolution, and produced a variety of AAV variants that can cross the blood-brain barrier in mice and monkeys.
These research results also show that the modification of the AAV capsid can improve the delivery efficiency of gene therapy and increase the specificity of neuron targeting.
This new AAV vector also provides a safer and more effective treatment option for brain diseases .

Viviana Gradinaru
Both Viviana Gradinaru is Zhang Feng graduated with a Ph.D. from the laboratory of Professor Karl Deisseroth , the father of optogenetics .
During the Ph.D. period, Viviana and Zhang Feng published several important papers in the field of optogenetics .
Paper link:
https://www.nature.com/articles/s41593-021-00969-4
New AVV variant breaks through the blood-brain barrier without being enriched in liver.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



